Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone
- PMID: 22184395
- PMCID: PMC3295561
- DOI: 10.1200/JCO.2010.33.7675
Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone
Abstract
Purpose: Persistent androgen signaling is implicated in castrate-resistant prostate cancer (CRPC) progression. This study aimed to evaluate androgen signaling in bone marrow-infiltrating cancer and testosterone in blood and bone marrow and to correlate with clinical observations.
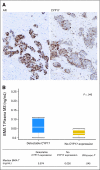
Patients and methods: This was an open-label, observational study of 57 patients with bone-metastatic CRPC who underwent transiliac bone marrow biopsy between October 2007 and March 2010. Patients received oral abiraterone acetate (1 g) once daily and prednisone (5 mg) twice daily. Androgen receptor (AR) and CYP17 expression were assessed by immunohistochemistry, testosterone concentration by mass spectrometry, AR copy number by polymerase chain reaction, and TMPRSS2-ERG status by fluorescent in situ hybridization in available tissues.
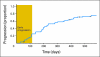
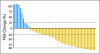
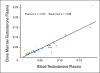
Results: Median overall survival was 555 days (95% CI, 440 to 965+ days). Maximal prostate-specific antigen decline ≥ 50% occurred in 28 (50%) of 56 patients. Homogeneous, intense nuclear expression of AR, combined with ≥ 10% CYP17 tumor expression, was correlated with longer time to treatment discontinuation (> 4 months) in 25 patients with tumor-infiltrated bone marrow samples. Pretreatment CYP17 tumor expression ≥ 10% was correlated with increased bone marrow aspirate testosterone. Blood and bone marrow aspirate testosterone concentrations declined to less than picograms-per-milliliter levels and remained suppressed at progression.
Conclusion: The observed pretreatment androgen-signaling signature is consistent with persistent androgen signaling in CRPC bone metastases. This is the first evidence that abiraterone acetate achieves sustained suppression of testosterone in both blood and bone marrow aspirate to less than picograms-per-milliliter levels. Potential admixture of blood with bone marrow aspirate limits our ability to determine the origin of measured testosterone.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Molecular states underlying androgen receptor activation: a framework for therapeutics targeting androgen signaling in prostate cancer.J Clin Oncol. 2012 Feb 20;30(6):644-6. doi: 10.1200/JCO.2011.39.1300. Epub 2011 Dec 19. J Clin Oncol. 2012. PMID: 22184375 No abstract available.
-
Role of androgens in abiraterone resistance.J Clin Oncol. 2012 Oct 1;30(28):3561-2: author reply 3562. doi: 10.1200/JCO.2012.42.6528. Epub 2012 Aug 20. J Clin Oncol. 2012. PMID: 22915660 No abstract available.
References
-
- Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin. 2002;52:154–179. - PubMed
-
- Geller J. Prolonging survival in metastatic prostate cancer: The case for adrenal androgens—Overview and summary of therapeutic controversies in prostatic cancer. J Clin Endocrinol Metab. 1995;80:1074–1078. - PubMed
-
- Mohler JL, Gregory CW, Ford OH, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. - PubMed
-
- Titus MA, Schell MJ, Lih FB, et al. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. - PubMed
-
- Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

