Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival
- PMID: 22184410
- PMCID: PMC3286213
- DOI: 10.1182/blood-2010-12-326843
Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival
Abstract
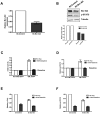
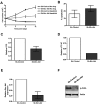
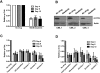
Recent evidence suggests chronic myeloid leukemia (CML) stem cells are insensitive to kinase inhibitors and responsible for minimal residual disease in treated patients. We investigated whether CML stem cells, in a transgenic mouse model of CML-like disease or derived from patients, are dependent on Bcr-Abl. In the transgenic model, after retransplantation, donor-derived CML stem cells in which Bcr-Abl expression had been induced and subsequently shut off were able to persist in vivo and reinitiate leukemia in secondary recipients on Bcr-Abl reexpression. Bcr-Abl knockdown in human CD34(+) CML cells cultured for 12 days in physiologic growth factors achieved partial inhibition of Bcr-Abl and downstream targets p-CrkL and p-STAT5, inhibition of proliferation and colony forming cells, but no reduction of input cells. The addition of dasatinib further inhibited p-CrkL and p-STAT5, yet only reduced input cells by 50%. Complete growth factor withdrawal plus dasatinib further reduced input cells to 10%; however, the surviving fraction was enriched for primitive leukemic cells capable of growth in a long-term culture-initiating cell assay and expansion on removal of dasatinib and addition of growth factors. Together, these data suggest that CML stem cell survival is Bcr-Abl kinase independent and suggest curative approaches in CML must focus on kinase-independent mechanisms of resistance.
Figures


References
-
- Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247(4944):824–830. - PubMed
-
- Koschmieder S, Gottgens B, Zhang P, et al. Inducible chronic phase of myeloid leukemia with expansion of hematopoietic stem cells in a transgenic model of BCR-ABL leukemogenesis. Blood. 2005;105(1):324–334. - PubMed
-
- Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356–361. - PubMed
-
- Notta F, Mullighan CG, Wang JC, et al. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature. 2011;469(7330):362–367. - PubMed
-
- Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

