Enhanced wound healing by recombinant Escherichia coli Nissle 1917 via human epidermal growth factor receptor in human intestinal epithelial cells: therapeutic implication using recombinant probiotics
- PMID: 22184415
- PMCID: PMC3294649
- DOI: 10.1128/IAI.05820-11
Enhanced wound healing by recombinant Escherichia coli Nissle 1917 via human epidermal growth factor receptor in human intestinal epithelial cells: therapeutic implication using recombinant probiotics
Abstract
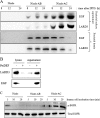
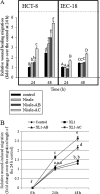
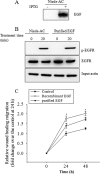
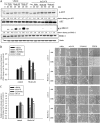
The gastrointestinal mucosa has a remarkable ability to repair damage with the support of epidermal growth factor (EGF), which stimulates epithelial migration and proliferative reepithelialization. For the treatment of mucosal injuries, it is important to develop efficient methods for the localized delivery of mucoactive biotherapeutics. The basic idea in the present study came from the assumption that an intestinal probiotic vehicle can carry and deliver key recombinant medicinal proteins to the injured epithelial target in patients with intestinal ulcerative diseases, including inflammatory bowel disease. The study was focused on the use of the safe probiotic E. coli Nissle 1917, which was constructed to secrete human EGF in conjunction with the lipase ABC transporter recognition domain (LARD). Using the in vitro physically wounded monolayer model, ABC transporter-mediated EGF secretion by probiotic E. coli Nissle 1917 was demonstrated to enhance the wound-healing migration of human enterocytes. Moreover, the epithelial wound closure was dependent on EGF receptor-linked activation, which exclusively involved the subsequent signaling pathway of the mitogen-activated protein kinase kinase (MEK) extracellular-related kinases 1 and 2 (ERK1/2). In particular, the migrating frontier of the wounded edge displayed the strongest EGF receptor-linked signaling activation in the presence of the recombinant probiotic. The present study provides a basis for the clinical application of human recombinant biotherapeutics via an efficient, safe probiotic vehicle.
Figures

Similar articles
-
Nononcogenic restoration of the intestinal barrier by E. coli-delivered human EGF.JCI Insight. 2019 Aug 22;4(16):e125166. doi: 10.1172/jci.insight.125166. JCI Insight. 2019. PMID: 31434808 Free PMC article.
-
Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling.J Biol Chem. 2004 Oct 22;279(43):44513-21. doi: 10.1074/jbc.M406253200. Epub 2004 Aug 16. J Biol Chem. 2004. PMID: 15316018
-
Epidermal Growth Factor Stimulates Transforming Growth Factor-Beta Receptor Type II Expression In Corneal Epithelial Cells.Sci Rep. 2019 May 30;9(1):8079. doi: 10.1038/s41598-019-42969-2. Sci Rep. 2019. PMID: 31147562 Free PMC article.
-
[Escherichia coli Nissle 1917 as safe vehicles for intestinal immune targeted therapy--a review].Wei Sheng Wu Xue Bao. 2013 Jun 4;53(6):538-44. Wei Sheng Wu Xue Bao. 2013. PMID: 24028055 Review. Chinese.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Lipase and protease double-deletion mutant of Pseudomonas fluorescens suitable for extracellular protein production.Appl Environ Microbiol. 2012 Dec;78(23):8454-62. doi: 10.1128/AEM.02476-12. Epub 2012 Oct 5. Appl Environ Microbiol. 2012. PMID: 23042178 Free PMC article.
-
Identification of the minimal region in lipase ABC transporter recognition domain of Pseudomonas fluorescens for secretion and fluorescence of green fluorescent protein.Microb Cell Fact. 2012 May 11;11:60. doi: 10.1186/1475-2859-11-60. Microb Cell Fact. 2012. PMID: 22578275 Free PMC article.
-
Nononcogenic restoration of the intestinal barrier by E. coli-delivered human EGF.JCI Insight. 2019 Aug 22;4(16):e125166. doi: 10.1172/jci.insight.125166. JCI Insight. 2019. PMID: 31434808 Free PMC article.
-
Recombinant porcine epidermal growth factor-secreting Lactococcus lactis promotes the growth performance of early-weaned piglets.BMC Vet Res. 2014 Aug 21;10:171. doi: 10.1186/s12917-014-0171-1. BMC Vet Res. 2014. PMID: 25142032 Free PMC article. Clinical Trial.
-
Regulation of the tagatose catabolic gene cluster and development of a tagatose-inducible expression system in the probiotic Escherichia coli Nissle 1917.Microb Cell Fact. 2025 Jul 4;24(1):158. doi: 10.1186/s12934-025-02771-4. Microb Cell Fact. 2025. PMID: 40615889 Free PMC article.
References
-
- Barrandon Y, Green H. 1987. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell 50:1131–1137 - PubMed
-
- Bickert T, et al. 2009. Probiotic Escherichia coli Nissle 1917 suppresses allergen-induced Th2 responses in the airways. Int. Arch. Allergy Immunol. 149:219–230 - PubMed
-
- Blay J, Brown KD. 1985. Epidermal growth factor promotes the chemotactic migration of cultured rat intestinal epithelial cells. J. Cell. Physiol. 124:107–112 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

