The antibacterial activity of LL-37 against Treponema denticola is dentilisin protease independent and facilitated by the major outer sheath protein virulence factor
- PMID: 22184422
- PMCID: PMC3294656
- DOI: 10.1128/IAI.05903-11
The antibacterial activity of LL-37 against Treponema denticola is dentilisin protease independent and facilitated by the major outer sheath protein virulence factor
Abstract
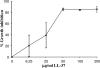
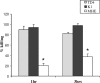
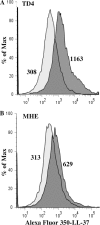
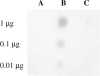
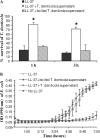
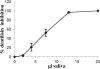
Host defense peptides are innate immune effectors that possess both bactericidal activities and immunomodulatory functions. Deficiency in the human host defense peptide LL-37 has previously been correlated with severe periodontal disease. Treponema denticola is an oral anaerobic spirochete closely associated with the pathogenesis of periodontal disease. The T. denticola major surface protein (MSP), involved in adhesion and cytotoxicity, and the dentilisin serine protease are key virulence factors of this organism. In this study, we examined the interactions between LL-37 and T. denticola. The three T. denticola strains tested were susceptible to LL-37. Dentilisin was found to inactivate LL-37 by cleaving it at the Lys, Phe, Gln, and Val residues. However, dentilisin deletion did not increase the susceptibility of T. denticola to LL-37. Furthermore, dentilisin activity was found to be inhibited by human saliva. In contrast, a deficiency of the T. denticola MSP increased resistance to LL-37. The MSP-deficient mutant bound less fluorescently labeled LL-37 than the wild-type strain. MSP demonstrated specific, dose-dependent LL-37 binding. In conclusion, though capable of LL-37 inactivation, dentilisin does not protect T. denticola from LL-37. Rather, the rapid, MSP-mediated binding of LL-37 to the treponemal outer sheath precedes cleavage by dentilisin. Moreover, in vivo, saliva inhibits dentilisin, thus preventing LL-37 restriction and ensuring its bactericidal and immunoregulatory activities.
Figures


Similar articles
-
Identification of the primary mechanism of complement evasion by the periodontal pathogen, Treponema denticola.Mol Oral Microbiol. 2011 Apr;26(2):140-9. doi: 10.1111/j.2041-1014.2010.00598.x. Epub 2010 Dec 3. Mol Oral Microbiol. 2011. PMID: 21375704 Free PMC article.
-
Crawling motility of Treponema denticola modulated by outer sheath protein.Microbiol Immunol. 2021 Dec;65(12):551-558. doi: 10.1111/1348-0421.12940. Epub 2021 Nov 15. Microbiol Immunol. 2021. PMID: 34499368
-
Localization and pathogenic role of the cysteine protease dentipain in Treponema denticola.Mol Oral Microbiol. 2023 Jun;38(3):212-223. doi: 10.1111/omi.12406. Epub 2023 Jan 28. Mol Oral Microbiol. 2023. PMID: 36641800 Free PMC article.
-
Approaches to Understanding Mechanisms of Dentilisin Protease Complex Expression in Treponema denticola.Front Cell Infect Microbiol. 2021 May 18;11:668287. doi: 10.3389/fcimb.2021.668287. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34084756 Free PMC article. Review.
-
Treponema denticola major surface protein (Msp): a key player in periodontal pathogenicity and immune evasion.Arch Microbiol. 2025 Jan 18;207(2):36. doi: 10.1007/s00203-024-04223-w. Arch Microbiol. 2025. PMID: 39825920 Review.
Cited by
-
The pathogenic mechanism of oral bacteria and treatment with inhibitors.Clin Exp Dent Res. 2022 Feb;8(1):439-448. doi: 10.1002/cre2.499. Epub 2021 Oct 9. Clin Exp Dent Res. 2022. PMID: 34626163 Free PMC article. Review.
-
Identification and characterization of fusolisin, the Fusobacterium nucleatum autotransporter serine protease.PLoS One. 2014 Oct 30;9(10):e111329. doi: 10.1371/journal.pone.0111329. eCollection 2014. PLoS One. 2014. PMID: 25357190 Free PMC article.
-
Significance of LL-37 on Immunomodulation and Disease Outcome.Biomed Res Int. 2020 May 16;2020:8349712. doi: 10.1155/2020/8349712. eCollection 2020. Biomed Res Int. 2020. PMID: 32509872 Free PMC article. Review.
-
Bioactive peptides in Endodontic infections: The Good, The Bad, and The Ugly.Probiotics Antimicrob Proteins. 2025 Jul 21. doi: 10.1007/s12602-025-10577-4. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40690088 Review.
-
Roles of cathelicidins in inflammation and bone loss.Odontology. 2014 Jul;102(2):137-46. doi: 10.1007/s10266-014-0167-0. Epub 2014 Jul 22. Odontology. 2014. PMID: 25047111 Review.
References
-
- Bachrach G, et al. 2006. Salivary LL-37 secretion in individuals with Down syndrome is normal. J. Dent. Res. 85:933–936 - PubMed
-
- Bergsson G, et al. 2009. LL-37 complexation with glycosaminoglycans in cystic fibrosis lungs inhibits antimicrobial activity, which can be restored by hypertonic saline. J. Immunol. 183:543–551 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

