Enterocytes' tight junctions: From molecules to diseases
- PMID: 22184542
- PMCID: PMC3241743
- DOI: 10.4291/wjgp.v2.i6.123
Enterocytes' tight junctions: From molecules to diseases
Abstract
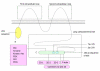
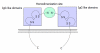
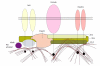
Tight junctions (TJs) are structures between cells where cells appear in the closest possible contact. They are responsible for sealing compartments when epithelial sheets are generated. They regulate the permeability of ions, (macro) molecules and cells via the paracellular pathway. Their structure at the electron microscopic level has been well known since the 1970s; however, only recently has their macromolecular composition been revealed. This review first examines the major macromolecular components of the TJs (occludin, claudins, junctional adhesion molecule and tricellulin) and then the associated macromolecules at the intracellular plaque [zonula occludens (ZO)-1, ZO-2, ZO-3, AF-6, cingulin, 7H6]. Emphasis is given to their interactions in order to begin to understand the mode of assembly of TJs. The functional significance of TJs is detailed and several mechanisms and factors involved are discussed briefly. Emphasis is given to the role of intestinal TJs and the alterations observed or speculated in diverse disease states. Specifically, intestinal TJs may exert a pathogenetic role in intestinal (inflammatory bowel disease, celiac disease) and extraintestinal diseases (diabetes type 1, food allergies, autoimmune diseases). Additionally, intestinal TJs may be secondarily disrupted during the course of diverse diseases, subsequently allowing the bacterial translocation phenomenon and promoting the systemic inflammatory response, which is often associated with clinical deterioration. The major questions in the field are highlighted.
Keywords: Claudins; Intestinal permeability; Junctional adhesion molecule; Occludin; Tight junctions; Tricellulin.
Figures


References
-
- Kachar B, Reese TS. Evidence for the lipidic nature of tight junction strands. Nature. 1982;296:464–466. - PubMed
-
- Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci. 1995;108(Pt 11):3443–3449. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

