High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses
- PMID: 22184635
- PMCID: PMC3260868
- DOI: 10.1084/jem.20101354
High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses
Abstract
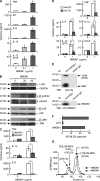
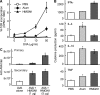
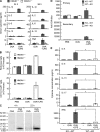
Alarmins are endogenous mediators capable of promoting the recruitment and activation of antigen-presenting cells (APCs), including dendritic cells (DCs), that can potentially alert host defense against danger signals. However, the relevance of alarmins to the induction of adaptive immune responses remains to be demonstrated. In this study, we report the identification of HMGN1 (high-mobility group nucleosome-binding protein 1) as a novel alarmin and demonstrate that it contributes to the induction of antigen-specific immune responses. HMGN1 induced DC maturation via TLR4 (Toll-like receptor 4), recruitment of APCs at sites of injection, and activation of NF-κB and multiple mitogen-activated protein kinases in DCs. HMGN1 promoted antigen-specific immune response upon co-administration with antigens, and Hmgn1(-/-) mice developed greatly reduced antigen-specific antibody and T cell responses when immunized with antigens in the presence of lipopolysaccharide (LPS). The impaired ability of Hmgn1(-/-) mice to mount antigen-specific immune responses was accompanied by both deficient DC recruitment at sites of immunization and reduced production of inflammatory cytokines. Bone marrow chimera experiments revealed that HMGN1 derived from nonleukocytes was critical for the induction of antigen-specific antibody and T cell responses. Thus, extracellular HMGN1 acts as a novel alarmin critical for LPS-induced development of innate and adaptive immune responses.
Figures

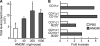


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

