Plasmodium falciparum population dynamics during the early phase of anti-malarial drug treatment in Tanzanian children with acute uncomplicated malaria
- PMID: 22185672
- PMCID: PMC3280947
- DOI: 10.1186/1475-2875-10-380
Plasmodium falciparum population dynamics during the early phase of anti-malarial drug treatment in Tanzanian children with acute uncomplicated malaria
Abstract
Background: This study aimed to explore Plasmodium falciparum population dynamics during the early phase of anti-malarial drug treatment with artemisinin-based combination therapy in children with clinical malaria in a high transmission area in Africa.
Methods: A total of 50 children aged 1-10 years with acute uncomplicated P. falciparum malaria in Bagamoyo District, Tanzania, were enrolled. Participants were hospitalized and received supervised standard treatment with artemether-lumefantrine according to body weight in six doses over 3 days. Blood samples were collected 11 times, i.e. at time of diagnosis (-2 h) and 0, 2, 4, 8, 16, 24, 36, 48, 60 and 72 h after initiation of treatment. Parasite population dynamics were assessed using nested polymerase chain reaction (PCR)-genotyping of merozoite surface protein (msp) 1 and 2.
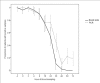
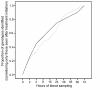
Results: PCR-analyses from nine sequential blood samples collected after initiation of treatment identified 20 and 21 additional genotypes in 15/50 (30%) and 14/50 (28%) children with msp1 and msp2, respectively, non-detectable in the pre-treatment samples (-2 and 0 h combined). Some 15/20 (75%) and 14/21 (67%) of these genotypes were identified within 24 h, whereas 17/20 (85%) and 19/21 (90%) within 48 h for msp1 and msp2, respectively. The genotype profile was diverse, and varied considerably over time both within and between patients, molecular markers and their respective families.
Conclusion: PCR analyses from multiple blood samples collected during the early treatment phase revealed a complex picture of parasite sub-populations. This underlines the importance of interpreting PCR-outcomes with caution and suggests that the present use of PCR-adjustment from paired blood samples in anti-malarial drug trials may overestimate assessment of drug efficacy in high transmission areas in Africa.The study is registered at http://www.clinicaltrials.gov with identifier NCT00336375.
Figures




References
-
- World Health Organization. Guidelines for the treatment of malaria. 2. Geneva: WHO; 2010. - PubMed
-
- Venture MfM, World Health Organization. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. Informal consultation organized by the MMV and cosponsored by the WHO 29-31 May 2007 Amsterdam; 2008.
-
- World Health Organization. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Geneva; 2003.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

