Structure, dynamics, and antimicrobial and immune modulatory activities of human LL-23 and its single-residue variants mutated on the basis of homologous primate cathelicidins
- PMID: 22185690
- PMCID: PMC3302206
- DOI: 10.1021/bi2016266
Structure, dynamics, and antimicrobial and immune modulatory activities of human LL-23 and its single-residue variants mutated on the basis of homologous primate cathelicidins
Abstract
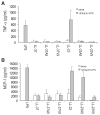
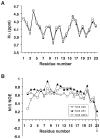
LL-23 is a natural peptide corresponding to the 23 N-terminal amino acid residues of human host defense cathelicidin LL-37. LL-23 demonstrated, compared to LL-37, a conserved ability to induce the chemokine MCP-1 in human peripheral blood mononuclear cells, a lack of ability to suppress induction of the pro-inflammatory cytokine TNF-α in response to bacterial lipopolysaccharides (LPS), and reduced antimicrobial activity. Heteronuclear multidimensional nuclear magnetic resonance (NMR) characterization of LL-23 revealed similar secondary structures and backbone dynamics in three membrane-mimetic micelles: SDS, dodecylphosphocholine (DPC), and dioctanoylphosphatidylglycerol. The NMR structure of LL-23 determined in perdeuterated DPC contained a unique serine that segregated the hydrophobic surface of the amphipathic helix into two domains. To improve our understanding, Ser9 of LL-23was changed to either Ala or Val on the basis of homologous primate cathelicidins. These changes made the hydrophobic surface of LL-23 continuous and enhanced antibacterial activity. While identical helical structures did not explain the altered activities, a reduced rate of hydrogen-deuterium exchange from LL-23 to LL-23A9 to LL-23V9 suggested a deeper penetration of LL-23V9 into the interior of the micelles, which correlated with enhanced activities. Moreover, these LL-23 variants had discrete immunomodulatory activities. Both restored the TNF-α dampening activity to the level of LL-37. Furthermore, LL-23A9, like LL-23, maintained superior protective MCP-1 production, while LL-23V9 was strongly immunosuppressive, preventing baseline MCP-1 induction and substantially reducing LPS-stimulated MCP-1 production. Thus, these LL-23 variants, designed on the basis of a structural hot spot, are promising immune modulators that are easier to synthesize and less toxic to mammalian cells than the parent peptide LL-37.
Figures





Similar articles
-
Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles.J Biol Chem. 2008 Nov 21;283(47):32637-43. doi: 10.1074/jbc.M805533200. Epub 2008 Sep 25. J Biol Chem. 2008. PMID: 18818205
-
Identification of chicken cathelicidin-2 core elements involved in antibacterial and immunomodulatory activities.Mol Immunol. 2009 Aug;46(13):2465-73. doi: 10.1016/j.molimm.2009.05.019. Epub 2009 Jun 12. Mol Immunol. 2009. PMID: 19524300
-
Alanine and Lysine Scans of the LL-37-Derived Peptide Fragment KR-12 Reveal Key Residues for Antimicrobial Activity.Chembiochem. 2018 May 4;19(9):931-939. doi: 10.1002/cbic.201700599. Epub 2018 Mar 30. Chembiochem. 2018. PMID: 29430821
-
High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments.Biochim Biophys Acta. 2014 Sep;1838(9):2160-72. doi: 10.1016/j.bbamem.2014.01.016. Epub 2014 Jan 23. Biochim Biophys Acta. 2014. PMID: 24463069 Free PMC article. Review.
-
The LL-37 domain: A clue to cathelicidin immunomodulatory response?Peptides. 2023 Jul;165:171011. doi: 10.1016/j.peptides.2023.171011. Epub 2023 Apr 15. Peptides. 2023. PMID: 37068711 Review.
Cited by
-
Structural Plasticity of LL-37 Indicates Elaborate Functional Adaptation Mechanisms to Bacterial Target Structures.Int J Mol Sci. 2021 May 14;22(10):5200. doi: 10.3390/ijms22105200. Int J Mol Sci. 2021. PMID: 34068993 Free PMC article. Review.
-
The Role of Antimicrobial Peptides in Influenza Virus Infection and Their Potential as Antiviral and Immunomodulatory Therapy.Pharmaceuticals (Basel). 2016 Sep 6;9(3):53. doi: 10.3390/ph9030053. Pharmaceuticals (Basel). 2016. PMID: 27608030 Free PMC article. Review.
-
Design and surface immobilization of short anti-biofilm peptides.Acta Biomater. 2017 Feb;49:316-328. doi: 10.1016/j.actbio.2016.11.061. Epub 2016 Nov 30. Acta Biomater. 2017. PMID: 27915018 Free PMC article.
-
Antimicrobial Peptides of the Cathelicidin Family: Focus on LL-37 and Its Modifications.Int J Mol Sci. 2025 Aug 21;26(16):8103. doi: 10.3390/ijms26168103. Int J Mol Sci. 2025. PMID: 40869425 Free PMC article. Review.
-
In Silico Structural Evaluation of Short Cationic Antimicrobial Peptides.Pharmaceutics. 2018 Jun 21;10(3):72. doi: 10.3390/pharmaceutics10030072. Pharmaceutics. 2018. PMID: 29933540 Free PMC article.
References
-
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. - PubMed
-
- Boman HG. Antibacterial peptides: basic facts and emerging concepts. J Inter Med. 2003;254:197–215. - PubMed
-
- Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr Opinion Immunol. 2002;14:96–102. - PubMed
-
- Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol. 2005;7:179–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

