Bmp signalling in filiform tongue papillae development
- PMID: 22186069
- PMCID: PMC3773933
- DOI: 10.1016/j.archoralbio.2011.11.014
Bmp signalling in filiform tongue papillae development
Abstract
Objective: Tongue papillae are critical organs in mastication. There are four different types of tongue papillae; fungiform, circumvallate, foliate, and filiform papillae. Unlike the other three taste papillae, non-gustatory papillae, filiform papillae cover the entire dorsal surface of the tongue and are important structures for the mechanical stress of sucking. Filiform papillae are further classified into two subtypes with different morphologies, depending on their location on the dorsum of the tongue. The filiform papillae at the intermolar eminence have pointed tips, whereas filiform papillae with rounded tips are found in other regions (anterior tongue). It remains unknown how the shape of each type of filiform papillae are determined during their development. Bmp signalling pathway has been known to regulate mechanisms that determine the shapes of many ectodermal organs. The aim of this study was to investigate the role of Bmp signalling in filiform papillae development.
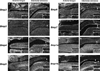
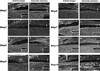
Design: Comparative in situ hybridization analysis of six Bmps (Bmp2-Bmp7) and two Bmpr genes (Bmpr1a and Bmpr1b) were carried out in filiform papillae development. We further examined tongue papillae in mice over-expressing Noggin under the keratin14 promoter (K14-Noggin).
Results: We identified a dynamic temporo-spatial expression of Bmps in filiform papillae development. The K14-Noggin mice showed pointed filiform papillae in regions of the tongue normally occupied by the rounded type.
Conclusions: Bmp signalling thus regulates the shape of filiform papillae.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Study by scanning electron microscopy of the morphogenesis of three types of lingual papilla in the rat.Anat Rec. 1997 Apr;247(4):528-41. doi: 10.1002/(SICI)1097-0185(199704)247:4<528::AID-AR12>3.0.CO;2-R. Anat Rec. 1997. PMID: 9096793
-
Bone morphogenetic proteins and noggin: inhibiting and inducing fungiform taste papilla development.Dev Biol. 2006 Sep 1;297(1):198-213. doi: 10.1016/j.ydbio.2006.05.022. Epub 2006 May 24. Dev Biol. 2006. PMID: 16828469
-
Study by scanning electron microscopy of the morphogenesis of three types of lingual papilla in the mouse.Acta Anat (Basel). 1996;157(1):41-52. doi: 10.1159/000147865. Acta Anat (Basel). 1996. PMID: 9096741
-
Regional regulation of Filiform tongue papillae development by Ikkα/Irf6.Dev Dyn. 2016 Sep;245(9):937-46. doi: 10.1002/dvdy.24427. Epub 2016 Jul 13. Dev Dyn. 2016. PMID: 27302476
-
Spacing patterns on tongue surface-gustatory papilla.Int J Dev Biol. 2004;48(2-3):157-61. doi: 10.1387/ijdb.15272380. Int J Dev Biol. 2004. PMID: 15272380 Review.
Cited by
-
Coevolutionary patterning of teeth and taste buds.Proc Natl Acad Sci U S A. 2015 Nov 3;112(44):E5954-62. doi: 10.1073/pnas.1514298112. Epub 2015 Oct 19. Proc Natl Acad Sci U S A. 2015. PMID: 26483492 Free PMC article.
-
Diode Laser in the Management of Foliate Papillitis - Two Case Reports.J Pharm Bioallied Sci. 2023 Jul;15(Suppl 1):S817-S820. doi: 10.4103/jpbs.jpbs_602_22. Epub 2023 Jul 5. J Pharm Bioallied Sci. 2023. PMID: 37654377 Free PMC article.
-
Taste papilla cell differentiation requires the regulation of secretory protein production by ALK3-BMP signaling in the tongue mesenchyme.Development. 2023 Sep 15;150(18):dev201838. doi: 10.1242/dev.201838. Epub 2023 Sep 25. Development. 2023. PMID: 37680190 Free PMC article.
-
Morphogenesis of fungiform papillae in developing miniature pigs.Heliyon. 2024 Jan 22;10(3):e24953. doi: 10.1016/j.heliyon.2024.e24953. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38314265 Free PMC article.
-
Ablation of NTPDase2+ cells inhibits the formation of filiform papillae in tongue tip.Animal Model Exp Med. 2018 Jul 28;1(2):143-151. doi: 10.1002/ame2.12021. eCollection 2018 Jun. Animal Model Exp Med. 2018. PMID: 30891559 Free PMC article.
References
-
- Iwasaki S, Okumura Y, Kumakura M. Ultrastructural study of the relationship between the morphogenesis of filiform papillae and the keratinization of the lingual epithelium in the mouse. Cells Tissues Organs. 1999;165(2):91–103. - PubMed
-
- Zhou Y, Liu HX, Mistretta CM. Bone morphogenetic proteins and noggin: inhibiting and inducing fungiform taste papilla development. Dev Biol. 2006;297(1):198–213. - PubMed
-
- Nie X. Apoptosis, proliferation and gene expression patterns in mouse developing tongue. Anat Embryol (Berl) 2005;210(2):125–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

