Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape
- PMID: 22186141
- PMCID: PMC3288154
- DOI: 10.1158/0008-5472.CAN-11-1620
Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape
Abstract
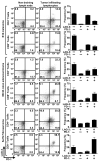
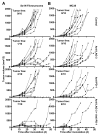
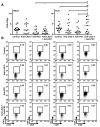
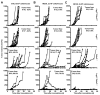
Inhibitory receptors on immune cells are pivotal regulators of immune escape in cancer. Among these inhibitory receptors, CTLA-4 (targeted clinically by ipilimumab) serves as a dominant off-switch while other receptors such as PD-1 and LAG-3 seem to serve more subtle rheostat functions. However, the extent of synergy and cooperative interactions between inhibitory pathways in cancer remain largely unexplored. Here, we reveal extensive coexpression of PD-1 and LAG-3 on tumor-infiltrating CD4(+) and CD8(+) T cells in three distinct transplantable tumors. Dual anti-LAG-3/anti-PD-1 antibody treatment cured most mice of established tumors that were largely resistant to single antibody treatment. Despite minimal immunopathologic sequelae in PD-1 and LAG-3 single knockout mice, dual knockout mice abrogated self-tolerance with resultant autoimmune infiltrates in multiple organs, leading to eventual lethality. However, Lag3(-/-)Pdcd1(-/-) mice showed markedly increased survival from and clearance of multiple transplantable tumors. Together, these results define a strong synergy between the PD-1 and LAG-3 inhibitory pathways in tolerance to both self and tumor antigens. In addition, they argue strongly that dual blockade of these molecules represents a promising combinatorial strategy for cancer.
Figures

References
-
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 29:235–71. - PubMed
-
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. - PubMed
-
- Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

