Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development
- PMID: 22186724
- PMCID: PMC3243091
- DOI: 10.1242/dev.067082
Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development
Erratum in
- Development. 2012 Feb;139(4):828
Abstract
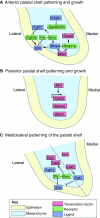
Mammalian palatogenesis is a highly regulated morphogenetic process during which the embryonic primary and secondary palatal shelves develop as outgrowths from the medial nasal and maxillary prominences, respectively, remodel and fuse to form the intact roof of the oral cavity. The complexity of control of palatogenesis is reflected by the common occurrence of cleft palate in humans. Although the embryology of the palate has long been studied, the past decade has brought substantial new knowledge of the genetic control of secondary palate development. Here, we review major advances in the understanding of the morphogenetic and molecular mechanisms controlling palatal shelf growth, elevation, adhesion and fusion, and palatal bone formation.
Figures



References
-
- Alappat S. R., Zhang Z., Suzuki K., Zhang X., Liu H., Jiang R., Yamada G., Chen Y. (2005). The cellular and molecular etiology of the cleft secondary palate in Fgf10 mutant mice. Dev. Biol. 277, 102-113 - PubMed
-
- Andl T., Ahn K., Kairo A., Chu E. Y., Wine-Lee L., Reddy S. T., Croft N. J., Cebra-Thomas J. A., Metzger D., Chambon P., et al. (2004). Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development 131, 2257-2268 - PubMed
-
- Barrow J. R., Stadler H. S., Capecchi M. R. (2000). Roles of Hoxa1 and Hoxa2 in patterning the early hindbrain of the mouse. Development 127, 933-944 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

