Shaping sound in space: the regulation of inner ear patterning
- PMID: 22186725
- PMCID: PMC3243092
- DOI: 10.1242/dev.067074
Shaping sound in space: the regulation of inner ear patterning
Erratum in
- Development. 2012 Feb;139(4):826
Abstract
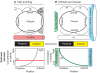
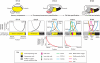
The inner ear is one of the most morphologically elaborate tissues in vertebrates, containing a group of mechanosensitive sensory organs that mediate hearing and balance. These organs are arranged precisely in space and contain intricately patterned sensory epithelia. Here, we review recent studies of inner ear development and patterning which reveal that multiple stages of ear development - ranging from its early induction from the embryonic ectoderm to the establishment of the three cardinal axes and the fine-grained arrangement of sensory cells - are orchestrated by gradients of signaling molecules.
Figures


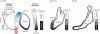
References
-
- Abello G., Khatri S., Radosevic M., Scotting P. J., Giraldez F., Alsina B. (2010). Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev. Biol. 339, 166-178 - PubMed
-
- Baker C. V., Bronner-Fraser M. (2001). Vertebrate cranial placodes I. Embryonic induction. Dev. Biol. 232, 1-61 - PubMed
-
- Bertrand N., Dahmane N. (2006). Sonic hedgehog signaling in forebrain development and its interactions with pathways that modify its effects. Trends Cell Biol. 16, 597-605 - PubMed
-
- Bok J., Bronner-Fraser M., Wu D. K. (2005). Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development 132, 2115-2124 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

