Phase I trial of "bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell" vaccine (FANG) in advanced cancer
- PMID: 22186789
- PMCID: PMC3293620
- DOI: 10.1038/mt.2011.269
Phase I trial of "bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell" vaccine (FANG) in advanced cancer
Abstract
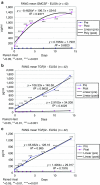
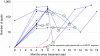
We performed a phase I trial of FANG vaccine, an autologous tumor-based product incorporating a plasmid encoding granulocyte-macrophage colony-stimulating factor (GMCSF) and a novel bifunctional short hairpin RNAi (bi-shRNAi) targeting furin convertase, thereby downregulating endogenous immunosuppressive transforming growth factors (TGF) β1 and β2. Patients with advanced cancer received up to 12 monthly intradermal injections of FANG vaccine (1 × 10(7) or 2.5 × 10(7) cells/ml injection). GMCSF, TGFβ1, TGFβ2, and furin proteins were quantified by enzyme-linked immunosorbent assay (ELISA). Safety and response were monitored. Vaccine manufacturing was successful in 42 of 46 patients of whom 27 received ≥1 vaccine. There were no treatment-related serious adverse events. Most common grade 1, 2 adverse events included local induration (n = 14) and local erythema (n = 11) at injection site. Post-transfection mean product expression GMCSF increased from 7.3 to 1,108 pg/10(6) cells/ml. Mean TGFβ1 and β2 effective target knockdown was 93.5 and 92.5% from baseline, respectively. Positive enzyme-linked immunospot (ELISPOT) response at month 4 was demonstrated in 9 of 18 patients serially assessed and correlated with survival duration from time of treatment (P = 0.025). Neither dose-adverse event nor dose-response relationship was noted. In conclusion, FANG vaccine was safe and elicited an immune response correlating with prolonged survival. Phase II assessment is justified.
Figures

References
-
- Smyth MJ, Dunn GP., and, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. - PubMed
-
- Schreiber RD, Old LJ., and, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. - PubMed
-
- Alard P, Clark SL., and, Kosiewicz MM. Deletion, but not anergy, is involved in TGF-beta-treated antigen-presenting cell-induced tolerance. Int Immunol. 2003;15:945–953. - PubMed
-
- Wahl SM, Swisher J, McCartney-Francis N., and, Chen W. TGF-beta: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J Leukoc Biol. 2004;76:15–24. - PubMed
-
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

