The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers
- PMID: 22186794
- PMCID: PMC3321594
- DOI: 10.1038/mt.2011.276
The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers
Abstract
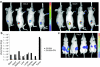
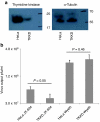
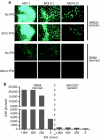
Oncolytic viruses are generally designed to be cancer selective on the basis of a single genetic mutation. JX-594 is a thymidine kinase (TK) gene-inactivated oncolytic vaccinia virus expressing granulocyte-macrophage colony-stimulating factor (GM-CSF) and lac-Z transgenes that is designed to destroy cancer cells through replication-dependent cell lysis and stimulation of antitumoral immunity. JX-594 has demonstrated a favorable safety profile and reproducible tumor necrosis in a variety of solid cancer types in clinical trials. However, the mechanism(s) responsible for its cancer-selectivity have not yet been well described. We analyzed the replication of JX-594 in three model systems: primary normal and cancer cells, surgical explants, and murine tumor models. JX-594 replication, transgene expression, and cytopathic effects were highly cancer-selective, and broad spectrum activity was demonstrated. JX-594 cancer-selectivity was multi-mechanistic; replication was activated by epidermal growth factor receptor (EGFR)/Ras pathway signaling, cellular TK levels, and cancer cell resistance to type-I interferons (IFNs). These findings confirm a large therapeutic index for JX-594 that is driven by common genetic abnormalities in human solid tumors. This appears to be the first description of multiple selectivity mechanisms, both inherent and engineered, for an oncolytic virus. These findings have implications for oncolytic viruses in general, and suggest that their cancer targeting is a complex and multifactorial process.
Figures
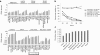


References
-
- Parato KA, Lichty BD., and, Bell JC. Diplomatic immunity: turning a foe into an ally. Curr Opin Mol Ther. 2009;11:13–21. - PubMed
-
- Bell JC, Lichty B., and, Stojdl D. Getting oncolytic virus therapies off the ground. Cancer Cell. 2003;4:7–11. - PubMed
-
- Liu TC, Hwang TH, Bell JC., and, Kirn DH. Development of targeted oncolytic virotherapeutics through translational research. Expert Opin Biol Ther. 2008;8:1381–1391. - PubMed
-
- Liu TC, Galanis E., and, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

