BRCA1 is an essential regulator of heart function and survival following myocardial infarction
- PMID: 22186889
- PMCID: PMC3247816
- DOI: 10.1038/ncomms1601
BRCA1 is an essential regulator of heart function and survival following myocardial infarction
Abstract
The tumour suppressor BRCA1 is mutated in familial breast and ovarian cancer but its role in protecting other tissues from DNA damage has not been explored. Here we show a new role for BRCA1 as a gatekeeper of cardiac function and survival. In mice, loss of BRCA1 in cardiomyocytes results in adverse cardiac remodelling, poor ventricular function and higher mortality in response to ischaemic or genotoxic stress. Mechanistically, loss of cardiomyocyte BRCA1 results in impaired DNA double-strand break repair and activated p53-mediated pro-apoptotic signalling culminating in increased cardiomyocyte apoptosis, whereas deletion of the p53 gene rescues BRCA1-deficient mice from cardiac failure. In human adult and fetal cardiac tissues, ischaemia induces double-strand breaks and upregulates BRCA1 expression. These data reveal BRCA1 as a novel and essential adaptive response molecule shielding cardiomyocytes from DNA damage, apoptosis and heart dysfunction. BRCA1 mutation carriers, in addition to risk of breast and ovarian cancer, may be at a previously unrecognized risk of cardiac failure.
Figures
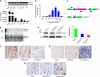
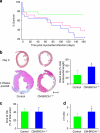

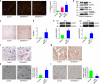
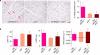
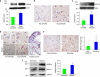
References
-
- Foulkes W. D. Inherited susceptibility to common cancers. N. Engl. J. Med. 359, 2143–2153 (2008). - PubMed
-
- Wooster R. & Weber B. L. Breast and ovarian cancer. N. Engl. J. Med. 348, 2339–2347 (2003). - PubMed
-
- Xu X. et al.. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat. Genet. 22, 37–43 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

