Promotion of plasma membrane repair by vitamin E
- PMID: 22186893
- PMCID: PMC3247818
- DOI: 10.1038/ncomms1594
Promotion of plasma membrane repair by vitamin E
Abstract
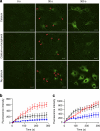
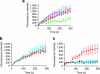
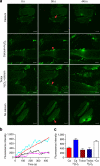
Severe vitamin E deficiency results in lethal myopathy in animal models. Membrane repair is an important myocyte response to plasma membrane disruption injury as when repair fails, myocytes die and muscular dystrophy ensues. Here we show that supplementation of cultured cells with α-tocopherol, the most common form of vitamin E, promotes plasma membrane repair. Conversely, in the absence of α-tocopherol supplementation, exposure of cultured cells to an oxidant challenge strikingly inhibits repair. Comparative measurements reveal that, to promote repair, an anti-oxidant must associate with membranes, as α-tocopherol does, or be capable of α-tocopherol regeneration. Finally, we show that myocytes in intact muscle cannot repair membranes when exposed to an oxidant challenge, but show enhanced repair when supplemented with vitamin E. Our work suggests a novel biological function for vitamin E in promoting myocyte plasma membrane repair. We propose that this function is essential for maintenance of skeletal muscle homeostasis.
Figures
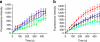


References
-
- Clarke M. S., Caldwell R. W., Chiao H., Miyake K. & McNeil P. L. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ. Res. 76, 927–934 (1995). - PubMed
-
- McNeil P. L. & Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology 96, 1238–1248 (1989). - PubMed
-
- McNeil P. L. & Ito S. Molecular traffic through plasma membrane disruptions of cells in vivo. J. Cell Sci. 96, 549–556 (1990). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

