Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis
- PMID: 22186970
- PMCID: PMC11412254
- DOI: 10.1039/c1np00068c
Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis
Abstract
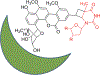
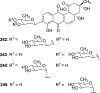
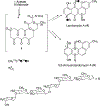
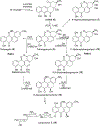
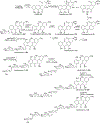
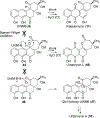
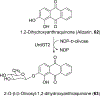
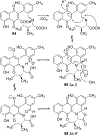
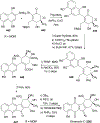
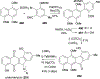
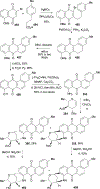
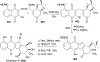
Covering: 1997 to 2010. The angucycline group is the largest group of type II PKS-engineered natural products, rich in biological activities and chemical scaffolds. This stimulated synthetic creativity and biosynthetic inquisitiveness. The synthetic studies used five different strategies, involving Diels-Alder reactions, nucleophilic additions, electrophilic additions, transition-metal mediated cross-couplings and intramolecular cyclizations to generate the angucycline frames. Biosynthetic studies were particularly intriguing when unusual framework rearrangements by post-PKS tailoring oxidoreductases occurred, or when unusual glycosylation reactions were involved in decorating the benz[a]anthracene-derived cores. This review follows our previous reviews, which were published in 1992 and 1997, and covers new angucycline group antibiotics published between 1997 and 2010. However, in contrast to the previous reviews, the main focus of this article is on new synthetic approaches and biosynthetic investigations, most of which were published between 1997 and 2010, but go beyond, e.g. for some biosyntheses all the way back to the 1980s, to provide the necessary context of information.
Figures







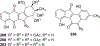










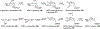
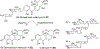



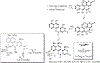



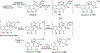
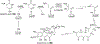


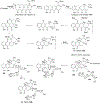
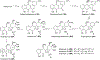
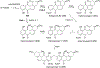
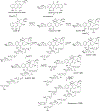


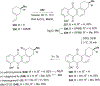

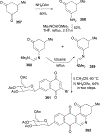

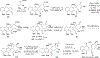
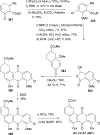
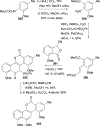
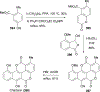



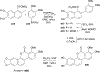
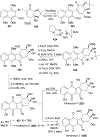

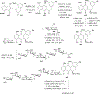



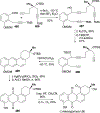
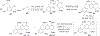




References
-
- Rohr J and Thiericke R, Nat. Prod. Rep, 1992, 9, 103–137. - PubMed
-
- Krohn K and Rohr J, Top. Curr. Chem, 1997, 188, 127–195.
-
- Yang X, Fu B and Yu B, J. Am. Chem. Soc, 2011, 133, 12433–12435. - PubMed
-
- Rohr J and Hertweck C, in Comprehensive Natural Products II – Chemistry and Biology, ed. Mander L and Liu H.-w., Elsevier, Oxford, 2010, pp. 227–303.
-
- Rohr J, Beale JM and Floss HG, J. Antibiot, 1989, 42, 1151–1157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

