Double-blind randomized trials of single-tablet ibuprofen/high-dose famotidine vs. ibuprofen alone for reduction of gastric and duodenal ulcers
- PMID: 22186979
- PMCID: PMC3321505
- DOI: 10.1038/ajg.2011.443
Double-blind randomized trials of single-tablet ibuprofen/high-dose famotidine vs. ibuprofen alone for reduction of gastric and duodenal ulcers
Abstract
Objectives: We performed two 24-week double-blind trials (REDUCE-1 and -2 (Registration Endoscopic Studies to Determine Ulcer Formation of HZT-501 Compared with Ibuprofen: Efficacy and Safety Studies)) to assess whether double-dose famotidine given in a single-tablet combination with ibuprofen (HZT-501) significantly reduces gastric and duodenal ulcers as compared with ibuprofen.
Methods: Patients (40-80 years) requiring daily non-steroidal anti-inflammatory drugs (NSAIDs) for ≥6 months with no prior ulcer complications, negative H. pylori stool test, and baseline endoscopy showing no ulcers and <5 erosions were randomly assigned in a 2:1 ratio to HZT-501 or identical-appearing ibuprofen 800 mg tablets thrice daily. Study endoscopies were done at 8, 16, and 24 weeks. After unblinding and initial analyses, 12 patients were found to be misclassified as having gastric ulcers based on the adjudication of endoscopy reports, and analyses were re-run.
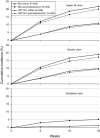
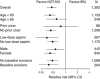
Results: In REDUCE-1, the primary end point analysis of gastric ulcers at 24 weeks with HZT-501 vs. ibuprofen was 12.7% vs. 22.9% (P=0.0044) in the post-adjudication analysis. In REDUCE-2, the primary end point analysis of upper gastrointestinal (GI) ulcers was 13.0% vs. 20.5% (P=0.0587) in the post-adjudication analysis. Prespecified pooled analyses showed significantly fewer gastric (12.5% vs. 20.7%) and duodenal ulcers (1.1% vs. 5.1%) with HZT-501 vs. ibuprofen. Proportional hazards analysis of multiple potential risk factors showed the risk ratio of upper GI ulcers with HZT-501 vs. ibuprofen was 0.46, 95% confidence interval was 0.34-0.61.
Conclusions: Combined results of the REDUCE studies indicate that double-dose famotidine plus ibuprofen, given as a combination tablet, decreases endoscopic upper GI ulcers as compared with ibuprofen alone.
Figures
Comment in
-
Editorial: single-tablet ibuprofen/double-dose famotidine for reduction of gastric and duodenal ulcers (REDUCE Trials): what can be reduced?Am J Gastroenterol. 2012 Mar;107(3):387-8. doi: 10.1038/ajg.2011.439. Am J Gastroenterol. 2012. PMID: 22388022
-
Single-tablet double-dose famotidine plus ibuprofen decreases endoscopic upper GI ulcers compared with ibuprofen alone.Evid Based Med. 2013 Feb;18(1):26-7. doi: 10.1136/ebmed-2012-100711. Epub 2012 Jul 7. Evid Based Med. 2013. PMID: 22773762 No abstract available.
References
-
- Kaufman DW, Kelly JP, Rosenberg L, et al. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287:337–344. - PubMed
-
- Paulose-Ram R, Hirsch R, Dillon C, et al. Prescription and non-prescription analgesic use among the US adult population: results from the third National Health and Nutrition Examination Survey (NHANES III) Pharmacoepidemiol Drug Safety. 2003;12:315–326. - PubMed
-
- Wilcox CM, Allison J, Benzuly K, et al. Consensus development conference on the use of nonsteroidal anti-inflammatory agents, including cyclooxygenase-2 enzyme inhibitors and aspirin. Clin Gastroenterol Hepatol. 2006;4:1082–1089. - PubMed
-
- Chan FKL, Abraham NS, Scheiman JS, et al. Management of patients on nonsteroidal anti-inflammatory drugs: a clinical practice recommendation from the first international working party on gastrointestinal and cardiovascular effects of nonsteroidal anti-inflammatory drugs and anti-platelet agents. Am J Gastroenterol. 2008;103:2908–2918. - PubMed
-
- Rostom A, Moayyedi P, Hunt R, et al. Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks. Aliment Pharmacol Ther. 2009;29:481–496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

