Characterization of SfmD as a Heme peroxidase that catalyzes the regioselective hydroxylation of 3-methyltyrosine to 3-hydroxy-5-methyltyrosine in saframycin A biosynthesis
- PMID: 22187429
- PMCID: PMC3281660
- DOI: 10.1074/jbc.M111.306316
Characterization of SfmD as a Heme peroxidase that catalyzes the regioselective hydroxylation of 3-methyltyrosine to 3-hydroxy-5-methyltyrosine in saframycin A biosynthesis
Abstract
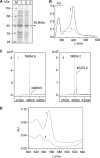
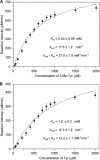
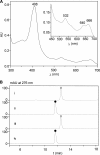
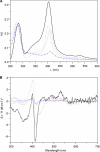
Saframycin A (SFM-A) is a potent antitumor antibiotic that belongs to the tetrahydroisoquinoline family. Biosynthetic studies have revealed that its unique pentacyclic core structure is derived from alanine, glycine, and non-proteinogenic amino acid 3-hydroxy-5-methyl-O-methyltyrosine (3-OH-5-Me-OMe-Tyr). SfmD, a hypothetical protein in the biosynthetic pathway of SFM-A, was hypothesized to be responsible for the generation of the 3-hydroxy group of 3-OH-5-Me-OMe-Tyr based on previously heterologous expression results. We now report the in vitro characterization of SfmD as a novel heme-containing peroxidase that catalyzes the hydroxylation of 3-methyltyrosine to 3-hydroxy-5-methyltyrosine using hydrogen peroxide as the oxidant. In addition, we elucidated the biosynthetic pathway of 3-OH-5-Me-OMe-Tyr by kinetic studies of SfmD in combination with biochemical assays of SfmM2, a methyltransferase within the same pathway. Furthermore, SacD, a counterpart of SfmD involved in safracin B biosynthesis, was also characterized as a heme-containing peroxidase, suggesting that SfmD-like heme-containing peroxidases may be commonly involved in the biosynthesis of SFM-A and its analogs. Finally, we found that the conserved motif HXXXC is crucial for heme binding using comparative UV-Vis and Magnetic Circular Dichroism (MCD) spectra studies of SfmD wild-type and mutants. Together, these findings expand the category of heme-containing peroxidases and set the stage for further mechanistic studies. In addition, this study has critical implications for delineating the biosynthetic pathway of other related tetrahydroisoquinoline family members.
Figures



References
-
- Scott J. D., Williams R. M. (2002) Chemistry and biology of the tetrahydroisoquinoline antitumor antibiotics. Chem. Rev. 102, 1669–1730 - PubMed
-
- Arai T., Takahashi K., Kubo A. (1977) New antibiotics saframycins A, B, C, D, and E. J. Antibiot. 30, 1015–1018 - PubMed
-
- Cuevas C., Francesch A. (2009) Development of Yondelis (trabectedin, ET-743). A semisynthetic process solves the supply problem. Nat. Prod. Rep. 26, 322–337 - PubMed
-
- Mikami Y., Takahashi K., Yazawa K., Arai T., Namikoshi M., Iwasaki S., Okuda S. (1985) Biosynthetic studies on saframycin A, a quinone antitumor antibiotic produced by Streptomyces lavendulae. J. Biol. Chem. 260, 344–348 - PubMed
-
- Palaniswamy V. A., Gould S. J. (1986) The incorporation of 3′-methyltyrosine and 5′-methyl DOPA into naphthyridinomycin. J. Am. Chem. Soc. 108, 5651–5652
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

