Axonal neuropathy-associated TRPV4 regulates neurotrophic factor-derived axonal growth
- PMID: 22187434
- PMCID: PMC3285368
- DOI: 10.1074/jbc.M111.316315
Axonal neuropathy-associated TRPV4 regulates neurotrophic factor-derived axonal growth
Abstract
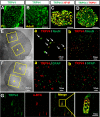
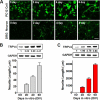
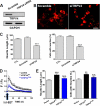
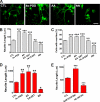
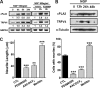
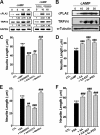
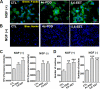
Spinal muscular atrophy and hereditary motor and sensory neuropathies are characterized by muscle weakness and atrophy caused by the degenerations of peripheral motor and sensory nerves. Recent advances in genetics have resulted in the identification of missense mutations in TRPV4 in patients with these hereditary neuropathies. Neurodegeneration caused by Ca(2+) overload due to the gain-of-function mutation of TRPV4 was suggested as the molecular mechanism for the neuropathies. Despite the importance of TRPV4 mutations in causing neuropathies, the precise role of TRPV4 in the sensory/motor neurons is unknown. Here, we report that TRPV4 mediates neurotrophic factor-derived neuritogenesis in developing peripheral neurons. TRPV4 was found to be highly expressed in sensory and spinal motor neurons in early development as well as in the adult, and the overexpression or chemical activation of TRPV4 was found to promote neuritogenesis in sensory neurons as well as PC12 cells, whereas its knockdown and pharmacologic inhibition had the opposite effect. More importantly, nerve growth factor or cAMP treatment up-regulated the expression of phospholipase A(2) and TRPV4. Neurotrophic factor-derived neuritogenesis appears to be regulated by the phospholipase A(2)-mediated TRPV4 pathway. These findings show that TRPV4 mediates neurotrophic factor-induced neuritogenesis in developing peripheral nerves. Because neurotrophic factors are essential for the maintenance of peripheral nerves, these findings suggest that aberrant TRPV4 activity may lead to some types of pathology of sensory and motor nerves.
Figures
Similar articles
-
The puzzle of TRPV4 channelopathies.EMBO Rep. 2013 Feb;14(2):152-63. doi: 10.1038/embor.2012.219. Epub 2013 Jan 11. EMBO Rep. 2013. PMID: 23306656 Free PMC article. Review.
-
Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy.BMC Neurol. 2007 May 23;7:11. doi: 10.1186/1471-2377-7-11. BMC Neurol. 2007. PMID: 17521436 Free PMC article.
-
Phenotypic spectrum and incidence of TRPV4 mutations in patients with inherited axonal neuropathy.Neurology. 2014 May 27;82(21):1919-26. doi: 10.1212/WNL.0000000000000450. Epub 2014 Apr 30. Neurology. 2014. PMID: 24789864
-
Incidence and Clinical Features of TRPV4-Linked Axonal Neuropathies in a USA Cohort of Charcot-Marie-Tooth Disease Type 2.Neuromolecular Med. 2020 Mar;22(1):68-72. doi: 10.1007/s12017-019-08564-4. Epub 2019 Aug 29. Neuromolecular Med. 2020. PMID: 31468327
-
TRPV4-mediated channelopathies.Channels (Austin). 2010 Jul-Aug;4(4):319-28. doi: 10.4161/chan.4.4.12905. Epub 2010 Jul 6. Channels (Austin). 2010. PMID: 20676052 Review.
Cited by
-
Electroacupuncture reduces cold stress-induced pain through microglial inactivation and transient receptor potential V1 in mice.Chin Med. 2021 Jun 3;16(1):43. doi: 10.1186/s13020-021-00451-0. Chin Med. 2021. PMID: 34082798 Free PMC article.
-
Combined Phenotypes of Spondylometaphyseal Dysplasia-Kozlowski Type and Charcot-Marie-Tooth Disease Type 2C Secondary to a TRPV4 Pathogenic Variant.Mol Syndromol. 2019 May;10(3):154-160. doi: 10.1159/000495778. Epub 2018 Dec 21. Mol Syndromol. 2019. PMID: 31191204 Free PMC article. Review.
-
Genome-wide DNA methylation profiling of human diabetic peripheral neuropathy in subjects with type 2 diabetes mellitus.Epigenetics. 2019 Aug;14(8):766-779. doi: 10.1080/15592294.2019.1615352. Epub 2019 May 28. Epigenetics. 2019. PMID: 31132961 Free PMC article.
-
Modification of TRPV4 activity by acetaminophen.Heliyon. 2020 Jan 31;6(1):e03301. doi: 10.1016/j.heliyon.2020.e03301. eCollection 2020 Jan. Heliyon. 2020. PMID: 32051870 Free PMC article.
-
The puzzle of TRPV4 channelopathies.EMBO Rep. 2013 Feb;14(2):152-63. doi: 10.1038/embor.2012.219. Epub 2013 Jan 11. EMBO Rep. 2013. PMID: 23306656 Free PMC article. Review.
References
-
- Reilly M. M., Murphy S. M., Laura M. (2011) Charcot-Marie-Tooth disease. J. Peripher. Nerv. Syst. 16, 1–14 - PubMed
-
- Drew A. P., Blair I. P., Nicholson G. A. (2011) Molecular genetics and mechanisms of disease in distal hereditary motor neuropathies. Insights directing future genetic studies. Curr. Mol. Med. 11, 650–665 - PubMed
-
- DeLong R., Siddique T. (1992) A large New England kindred with autosomal dominant neurogenic scapuloperoneal amyotrophy with unique features. Arch. Neurol. 49, 905–908 - PubMed
-
- Shy M. E., Patzkó A. (2011) Axonal Charcot-Marie-Tooth disease. Curr. Opin. Neurol. 24, 475–483 - PubMed
-
- Auer-Grumbach M., Olschewski A., Papi L., Kremer H., McEntagart M. E., Uhrig S., Fischer C., Fröhlich E., Bálint Z., Tang B., Strohmaier H., Lochmüller H., Schlotter-Weigel B., Senderek J., Krebs A., Dick K. J., Petty R., Longman C., Anderson N. E., Padberg G. W., Schelhaas H. J., van Ravenswaaij-Arts C. M., Pieber T. R., Crosby A. H., Guelly C. (2010) Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA, and HMSN2C. Nat. Genet. 42, 160–164 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

