Up-regulation of Cavβ3 subunit in primary sensory neurons increases voltage-activated Ca2+ channel activity and nociceptive input in neuropathic pain
- PMID: 22187436
- PMCID: PMC3285367
- DOI: 10.1074/jbc.M111.310110
Up-regulation of Cavβ3 subunit in primary sensory neurons increases voltage-activated Ca2+ channel activity and nociceptive input in neuropathic pain
Abstract
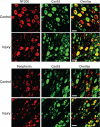
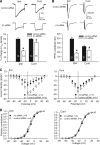
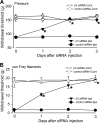
High voltage-activated calcium channels (HVACCs) are essential for synaptic and nociceptive transmission. Although blocking HVACCs can effectively reduce pain, this treatment strategy is associated with intolerable adverse effects. Neuronal HVACCs are typically composed of α(1), β (Cavβ), and α(2)δ subunits. The Cavβ subunit plays a crucial role in the membrane expression and gating properties of the pore-forming α(1) subunit. However, little is known about how nerve injury affects the expression and function of Cavβ subunits in primary sensory neurons. In this study, we found that Cavβ(3) and Cavβ(4) are the most prominent subtypes expressed in the rat dorsal root ganglion (DRG) and dorsal spinal cord. Spinal nerve ligation (SNL) in rats significantly increased mRNA and protein levels of the Cavβ(3), but not Cavβ(4), subunit in the DRG. SNL also significantly increased HVACC currents in small DRG neurons and monosynaptic excitatory postsynaptic currents of spinal dorsal horn neurons evoked from the dorsal root. Intrathecal injection of Cavβ(3)-specific siRNA significantly reduced HVACC currents in small DRG neurons and the amplitude of monosynaptic excitatory postsynaptic currents of dorsal horn neurons in SNL rats. Furthermore, intrathecal treatment with Cavβ(3)-specific siRNA normalized mechanical hyperalgesia and tactile allodynia caused by SNL but had no significant effect on the normal nociceptive threshold. Our findings provide novel evidence that increased expression of the Cavβ(3) subunit augments HVACC activity in primary sensory neurons and nociceptive input to dorsal horn neurons in neuropathic pain. Targeting the Cavβ(3) subunit at the spinal level represents an effective strategy for treating neuropathic pain.
Figures




References
-
- Dworkin R. H., O'Connor A. B., Backonja M., Farrar J. T., Finnerup N. B., Jensen T. S., Kalso E. A., Loeser J. D., Miaskowski C., Nurmikko T. J., Portenoy R. K., Rice A. S., Stacey B. R., Treede R. D., Turk D. C., Wallace M. S. (2007) Pharmacologic management of neuropathic pain. Evidence-based recommendations. Pain 132, 237–251 - PubMed
-
- Campero M., Serra J., Marchettini P., Ochoa J. L. (1998) Ectopic impulse generation and autoexcitation in single myelinated afferent fibers in patients with peripheral neuropathy and positive sensory symptoms. Muscle Nerve 21, 1661–1667 - PubMed
-
- Gracely R. H., Lynch S. A., Bennett G. J. (1992) Painful neuropathy. Altered central processing maintained dynamically by peripheral input. Pain 51, 175–194 - PubMed
-
- Catterall W. A., Few A. P. (2008) Calcium channel regulation and presynaptic plasticity. Neuron 59, 882–901 - PubMed
-
- Catterall W. A. (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

