Olfactomedin 4 defines a subset of human neutrophils
- PMID: 22187488
- PMCID: PMC3289394
- DOI: 10.1189/jlb.0811417
Olfactomedin 4 defines a subset of human neutrophils
Abstract
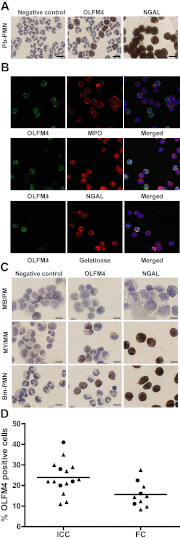
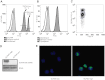
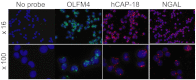
OLFM4 was identified initially as a gene highly induced in myeloid stem cells by G-CSF treatment. A bioinformatics method using a global meta-analysis of microarray data predicted that OLFM4 would be associated with specific granules in human neutrophils. Subcellular fractionation of peripheral blood neutrophils demonstrated complete colocalization of OLFM4 with the specific granule protein NGAL, and stimulation of neutrophils with PMA resulted in corelease of NGAL and OLFM4, proving that OLFM4 is a genuine constituent of neutrophil-specific granules. In accordance with this, OLFM4 mRNA peaked at the MY/MM stage of maturation. OLFM4 was, however, present in only 20-25% of peripheral blood neutrophils, as determined by immunocytochemistry and flow cytometry, whereas mRNA for OLFM4 was present in all MY/MM, indicating post-transcriptional regulation as a basis for the heterogeneous expression of OLFM4 protein.
Figures

Similar articles
-
A neutrophil subset defined by intracellular olfactomedin 4 is associated with mortality in sepsis.Am J Physiol Lung Cell Mol Physiol. 2021 May 1;320(5):L892-L902. doi: 10.1152/ajplung.00090.2020. Epub 2020 Dec 23. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33355521 Free PMC article. Clinical Trial.
-
Olfactomedin 4 is a novel target gene of retinoic acids and 5-aza-2'-deoxycytidine involved in human myeloid leukemia cell growth, differentiation, and apoptosis.Blood. 2010 Dec 2;116(23):4938-47. doi: 10.1182/blood-2009-10-246439. Epub 2010 Aug 19. Blood. 2010. PMID: 20724538 Free PMC article.
-
Longitudinal characterization of olfactomedin-4 expressing neutrophils in pediatric patients undergoing bone marrow transplantation.PLoS One. 2020 May 29;15(5):e0233738. doi: 10.1371/journal.pone.0233738. eCollection 2020. PLoS One. 2020. PMID: 32470072 Free PMC article.
-
Olfactomedin 4, a novel marker for the differentiation and progression of gastrointestinal cancers.Neoplasma. 2011;58(1):9-13. doi: 10.4149/neo_2011_01_9. Neoplasma. 2011. PMID: 21067260 Review.
-
Olfactomedin-4 in digestive diseases: A mini-review.World J Gastroenterol. 2018 May 7;24(17):1881-1887. doi: 10.3748/wjg.v24.i17.1881. World J Gastroenterol. 2018. PMID: 29740203 Free PMC article. Review.
Cited by
-
Olfactomedin 4 as a novel loop of Henle-specific acute kidney injury biomarker.Physiol Rep. 2022 Sep;10(18):e15453. doi: 10.14814/phy2.15453. Physiol Rep. 2022. PMID: 36117416 Free PMC article.
-
Olfactomedin 4 Is a Biomarker for the Severity of Infectious Diseases.Open Forum Infect Dis. 2022 Feb 8;9(4):ofac061. doi: 10.1093/ofid/ofac061. eCollection 2022 Apr. Open Forum Infect Dis. 2022. PMID: 35291445 Free PMC article. Review.
-
Plasma levels of OLFM4 in normals and patients with gastrointestinal cancer.J Cell Mol Med. 2015 Dec;19(12):2865-73. doi: 10.1111/jcmm.12679. Epub 2015 Sep 28. J Cell Mol Med. 2015. PMID: 26416558 Free PMC article.
-
Quantitative proteomics reveals tissue-specific, infection-induced and species-specific neutrophil protein signatures.Sci Rep. 2024 Mar 12;14(1):5966. doi: 10.1038/s41598-024-56163-6. Sci Rep. 2024. PMID: 38472281 Free PMC article.
-
Olfactomedin 4-Positive Neutrophils Are Upregulated after Hemorrhagic Shock.Am J Respir Cell Mol Biol. 2021 Feb;64(2):216-223. doi: 10.1165/rcmb.2020-0276OC. Am J Respir Cell Mol Biol. 2021. PMID: 33253592 Free PMC article.
References
-
- Zhang X., Huang Q., Yang Z., Li Y., Li C.Y. (2004) GW112, a novel antiapoptotic protein that promotes tumor growth. Cancer Res. 64, 2474–2481 - PubMed
-
- Zhang J., Liu W. L., Tang D.C., Chen L., Wang M., Pack S. D., Zhuang Z., Rodgers G. P. (2002) Identification and characterization of a novel member of olfactomedin-related protein family, hGC-1, expressed during myeloid lineage development. Gene 283, 83–93 - PubMed
-
- Oue N., Sentani K., Noguchi T., Ohara S., Sakamoto N., Hayashi T., Anami K., Motoshita J., Ito M., Tanaka S., Yoshida K., Yasui W. (2009) Serum olfactomedin 4 (GW112, hGC-1) in combination with Reg IV is a highly sensitive biomarker for gastric cancer patients. Int. J. Cancer 125, 2383–2392 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

