Ovarian surface epithelium in patients with severe ovarian infertility: a potential source of cells expressing markers of pluripotent/multipotent stem cells
- PMID: 22187524
- PMCID: PMC3237017
- DOI: 10.1155/2011/381928
Ovarian surface epithelium in patients with severe ovarian infertility: a potential source of cells expressing markers of pluripotent/multipotent stem cells
Abstract
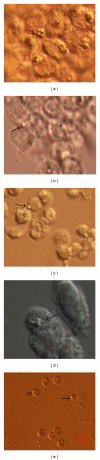
The aim of this study was to confirm the presence of stem cells in the ovarian surface epithelium of patients with premature ovarian failure and no mature follicles and oocytes. In these patients, small round cells of unknown origin expressing SOX-2 marker of pluripotency were observed among the epithelial cells just after the ovarian surface epithelium scraping. These cells were an integral part of the ovarian surface epithelium. When the scraped cells were cultured in a medium with added follicular fluid to provide some ovarian niche, primitive oocyte-like cells and typical round-shaped cell clusters positively stained on alkaline phosphatase, and markers of pluripotency, such as SOX-2 and SSEA-4, were developed. These markers were expressed early and also later in the culture. Single oocyte-like cells expressed genes OCT4A, SOX-2, NANOG, NANOS, STELLA, CD9, LIN28, KLF4, GDF3, and MYC, characteristic for pluripotent stem cells. The results of this study confirmed the presence of putative stem cells in the ovarian surface epithelium of these patients and provided some basis to create a stem cell line in the future.
Figures









Similar articles
-
Expression of pluripotency and oocyte-related genes in single putative stem cells from human adult ovarian surface epithelium cultured in vitro in the presence of follicular fluid.Biomed Res Int. 2013;2013:861460. doi: 10.1155/2013/861460. Epub 2013 Feb 28. Biomed Res Int. 2013. PMID: 23555100 Free PMC article.
-
Germline cells in ovarian surface epithelium of mammalians: a promising notion.Reprod Biol Endocrinol. 2012 Dec 17;10:112. doi: 10.1186/1477-7827-10-112. Reprod Biol Endocrinol. 2012. PMID: 23245287 Free PMC article. Review.
-
Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes.Differentiation. 2008 Oct;76(8):843-56. doi: 10.1111/j.1432-0436.2008.00268.x. Epub 2008 Apr 29. Differentiation. 2008. PMID: 18452550
-
Small putative NANOG, SOX2, and SSEA-4-positive stem cells resembling very small embryonic-like stem cells in sections of ovarian tissue in patients with ovarian cancer.J Ovarian Res. 2016 Mar 3;9:12. doi: 10.1186/s13048-016-0221-3. J Ovarian Res. 2016. PMID: 26940129 Free PMC article.
-
FSH-FSHR3-stem cells in ovary surface epithelium: basis for adult ovarian biology, failure, aging, and cancer.Reproduction. 2015 Jan;149(1):R35-48. doi: 10.1530/REP-14-0220. Epub 2014 Sep 30. Reproduction. 2015. PMID: 25269615 Review.
Cited by
-
Expression of pluripotency and oocyte-related genes in single putative stem cells from human adult ovarian surface epithelium cultured in vitro in the presence of follicular fluid.Biomed Res Int. 2013;2013:861460. doi: 10.1155/2013/861460. Epub 2013 Feb 28. Biomed Res Int. 2013. PMID: 23555100 Free PMC article.
-
Technical challenges and limitations of current mouse models of ovarian cancer.J Ovarian Res. 2012 Nov 29;5(1):39. doi: 10.1186/1757-2215-5-39. J Ovarian Res. 2012. PMID: 23190474 Free PMC article.
-
Hedgehog pathway inhibition causes primary follicle atresia and decreases female germline stem cell proliferation capacity or stemness.Stem Cell Res Ther. 2019 Jul 5;10(1):198. doi: 10.1186/s13287-019-1299-5. Stem Cell Res Ther. 2019. PMID: 31277696 Free PMC article.
-
Germline cells in ovarian surface epithelium of mammalians: a promising notion.Reprod Biol Endocrinol. 2012 Dec 17;10:112. doi: 10.1186/1477-7827-10-112. Reprod Biol Endocrinol. 2012. PMID: 23245287 Free PMC article. Review.
-
β-catenin/Tcf-signaling appears to establish the murine ovarian surface epithelium (OSE) and remains active in selected postnatal OSE cells.BMC Dev Biol. 2012 Jun 8;12:17. doi: 10.1186/1471-213X-12-17. BMC Dev Biol. 2012. PMID: 22682531 Free PMC article.
References
-
- Auersperg N, Siemens CH, Myrdal SE. Human ovarian surface epithelium in primary culture. In Vitro. 1984;20(10):743–755. - PubMed
-
- Siemens CH, Auersperg N. Serial propagation of human ovarian surface epithelium in tissue culture. Journal of Cellular Physiology. 1988;134(3):347–356. - PubMed
-
- Kruk PA, Maines-Bandiera SL, Auersperg N. A simplified method to culture human ovarian surface epithelium. Laboratory Investigation. 1990;63(1):132–136. - PubMed
-
- Gubbay O, Guo W, Rae MT, et al. Anti-inflammatory and proliferative responses in human and ovine ovarian surface epithelial cells. Reproduction. 2004;128(5):607–614. - PubMed
-
- Rae MT, Niven D, Critchley HOD, Harlow CR, Hillier SG. Antiinflammatory steroid action in human ovarian surface epithelial cells. Journal of Clinical Endocrinology and Metabolism. 2004;89(9):4538–4544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

