High-sensitivity nanosensors for biomarker detection
- PMID: 22187721
- PMCID: PMC3629948
- DOI: 10.1039/c1cs15238f
High-sensitivity nanosensors for biomarker detection
Abstract
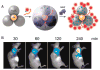
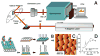
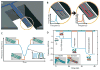
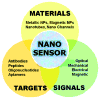
High sensitivity nanosensors utilize optical, mechanical, electrical, and magnetic relaxation properties to push detection limits of biomarkers below previously possible concentrations. The unique properties of nanomaterials and nanotechnology are exploited to design biomarker diagnostics. High-sensitivity recognition is achieved by signal and target amplification along with thorough pre-processing of samples. In this tutorial review, we introduce the type of detection signals read by nanosensors to detect extremely small concentrations of biomarkers and provide distinctive examples of high-sensitivity sensors. The use of such high-sensitivity nanosensors can offer earlier detection of disease than currently available to patients and create significant improvements in clinical outcomes.
Figures








References
-
- Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L. Nat Rev Cancer. 2003;3:243–252. - PubMed
-
- Ludwig JA, Weinstein JN. Nat Rev Cancer. 2005;5:845–856. - PubMed
-
- Rifai N, Gillette MA, Carr SA. Nat Biotechnol. 2006;24:971–983. - PubMed
-
- Nam JM, Thaxton CS, Mirkin CA. Science. 2003;301:1884–1886. - PubMed
-
- Cheng MMC, Cuda G, Bunimovich YL, Gaspari M, Heath JR, Hill HD, Mirkin CA, Nijdam AJ, Terracciano R, Thundat T, Ferrari M. Curr Opin Chem Biol. 2006;10:11–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

