Access of inhibitory neurosteroids to the NMDA receptor
- PMID: 22188257
- PMCID: PMC3417430
- DOI: 10.1111/j.1476-5381.2011.01816.x
Access of inhibitory neurosteroids to the NMDA receptor
Abstract
Background and purpose: NMDA receptors are glutamatergic ionotropic receptors involved in excitatory neurotransmission, synaptic plasticity and excitotoxic cell death. Many allosteric modulators can influence the activity of these receptors positively or negatively, with behavioural consequences. 20-Oxo-5β-pregnan-3α-yl sulphate (pregnanolone sulphate; PA-6) is an endogenous neurosteroid that inhibits NMDA receptors and is neuroprotective. We tested the hypothesis that the interaction of PA-6 with the plasma membrane is critical for its inhibitory effect at NMDA receptors.
Experimental approach: Electrophysiological recordings and live microscopy were performed on heterologous HEK293 cells expressing GluN1/GluN2B receptors and cultured rat hippocampal neurons.
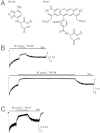
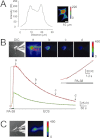
Key results: Our experiments showed that the kinetics of the steroid inhibition were slow and not typical of drug-receptor interaction in an aqueous solution. In addition, the recovery from steroid inhibition was accelerated by β- and γ-cyclodextrin. Values of IC(50) assessed for novel synthetic C3 analogues of PA-6 differed by more than 30-fold and were positively correlated with the lipophilicity of the PA-6 analogues. Finally, the onset of inhibition induced by C3 analogues of PA-6 ranged from use-dependent to use-independent. The onset and offset of cell staining by fluorescent analogues of PA-6 were slower than those of steroid-induced inhibition of current responses mediated by NMDA receptors.
Conclusion and implications: We conclude that steroid accumulation in the plasma membrane is the route by which it accesses a binding site on the NMDA receptor. Thus, our results provide a possible structural framework for pharmacologically targeting the transmembrane domains of the receptor.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures
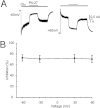
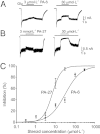



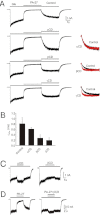

References
-
- Adam JM, Bennett DJ, Bom A, Clark JK, Feilden H, Hutchinson EJ, et al. Cyclodextrin-derived host molecules as reversal agents for the neuromuscular blocker rocuronium bromide: synthesis and structure-activity relationships. J Med Chem. 2002;45:1806–1816. - PubMed
-
- Besshoh S, Chen S, Brown IR, Gurd JW. Developmental changes in the association of NMDA receptors with lipid rafts. J Neurosci Res. 2007;85:1876–1883. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

