Concomitant facilitation of GABAA receptors and KV7 channels by the non-opioid analgesic flupirtine
- PMID: 22188423
- PMCID: PMC3419907
- DOI: 10.1111/j.1476-5381.2011.01821.x
Concomitant facilitation of GABAA receptors and KV7 channels by the non-opioid analgesic flupirtine
Abstract
Background and purpose: Flupirtine is a non-opioid analgesic that has been in clinical use for more than 20 years. It is characterized as a selective neuronal potassium channel opener (SNEPCO). Nevertheless, its mechanisms of action remain controversial and are the purpose of this study.
Experimental approach: Effects of flupirtine on native and recombinant voltage- and ligand-gated ion channels were explored in patch-clamp experiments using the following experimental systems: recombinant K(IR)3 and K(V)7 channels and α3β4 nicotinic acetylcholine receptors expressed in tsA 201 cells; native voltage-gated Na(+), Ca(2+), inward rectifier K(+), K(V)7 K(+), and TRPV1 channels, as well as GABA(A), glycine, and ionotropic glutamate receptors expressed in rat dorsal root ganglion, dorsal horn and hippocampal neurons.
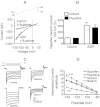
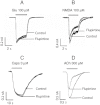
Key results: Therapeutic flupirtine concentrations (≤10 µM) did not affect voltage-gated Na(+) or Ca(2+) channels, inward rectifier K(+) channels, nicotinic acetylcholine receptors, glycine or ionotropic glutamate receptors. Flupirtine shifted the gating of K(V)7 K(+) channels to more negative potentials and the gating of GABA(A) receptors to lower GABA concentrations. These latter effects were more pronounced in dorsal root ganglion and dorsal horn neurons than in hippocampal neurons. In dorsal root ganglion and dorsal horn neurons, the facilitatory effect of therapeutic flupirtine concentrations on K(V)7 channels and GABA(A) receptors was comparable, whereas in hippocampal neurons the effects on K(V)7 channels were more pronounced.
Conclusions and implications: These results indicate that flupirtine exerts its analgesic action by acting on both GABA(A) receptors and K(V)7 channels.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures
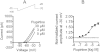

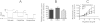
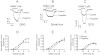
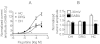
References
-
- Clark RJ, Fischer H, Nevin ST, Adams DJ, Craik DJ. The synthesis, structural characterization, and receptor specificity of the alpha-conotoxin Vc1.1. J Biol Chem. 2006;281:23254–23263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

