Amyloid generation and dysfunctional immunoproteasome activation with disease progression in animal model of familial Alzheimer's disease
- PMID: 22188425
- PMCID: PMC8057644
- DOI: 10.1111/j.1750-3639.2011.00560.x
Amyloid generation and dysfunctional immunoproteasome activation with disease progression in animal model of familial Alzheimer's disease
Abstract
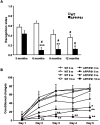
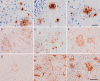
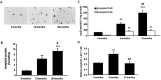
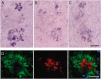
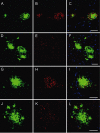
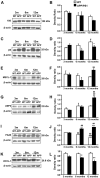
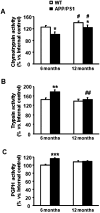
Double-transgenic amyloid precursor protein/presenilin 1 (APP/PS1) mice express a chimeric mouse/human APP bearing the Swedish mutation (Mo/HuAPP695swe) and a mutant human PS1-dE9 both causative of familial Alzheimer's disease (FAD). Transgenic mice show impaired memory and learning performance from the age of 6 months onwards. Double-transgenic APP/PS1 mice express altered APP and PS1 mRNAs and proteins, reduced β-secretase 1 (BACE1) mRNA and normal BACE1 protein, all of which suggest a particular mechanism of amyloidogenesis when compared with sporadic AD. The first β-amyloid plaques in APP/PS1 mice appear at 3 months, and they increase in number and distribution with disease progression in parallel with increased levels of brain soluble β-amyloid 1-42 and 1-40, but also with reduced 1-42/1-40 ratio with age. Amyloid deposition in plaques is accompanied by altered mitochondria and increased oxidative damage, post-translational modifications and accumulation of altered proteins at the dystrophic neurites surrounding plaques. Degradation pathways are also modified with disease progression including activation of the immunoproteasome together with variable alterations of the different protease activities of the ubiquitin-proteasome system. Present observations show modifications in the production of β-amyloid and activation and malfunction of the subcellular degradation pathways that have general implications in the pathogenesis of AD and more particularly in specificities of FAD amyloidogenesis.
© 2011 The Authors; Brain Pathology © 2011 International Society of Neuropathology.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



References
-
- Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ et al (2006) Phosphorylation of Ser‐129 is the dominant pathological modification of α‐synuclein in familial and sporadic Lewy body disease. J Biol Chem 281:29739–29752. - PubMed
-
- Blanchard V, Moussaoui S, Czech C, Touchet N, Bonici B, Planche M et al (2003) Time sequence of maturation of dystrophic neurites associated with Abeta deposits in APP/PS1 transgenic mice. Exp Neurol 184:247–263. - PubMed
-
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T et al (1996) Familial Alzheimer's disease‐linked presenilin 1 variants elevate Abeta1‐42/1‐40 ratio in vitro and in vivo. Neuron 17:1005–1013. - PubMed
-
- Botchler M, Ditzel Groll M, Hartmann C, Huber R (1999) The proteasome. Annu Rev Biophys Biomol Struct 28:295–317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

