Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue
- PMID: 22188562
- PMCID: PMC3411362
- DOI: 10.1089/scd.2011.0674
Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue
Abstract
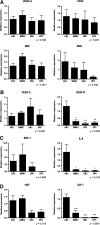
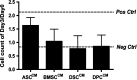
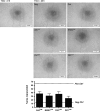
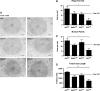
Human adult mesenchymal stem cells (MSCs) support the engineering of functional tissue constructs by secreting angiogenic and cytoprotective factors, which act in a paracrine fashion to influence cell survival and vascularization. MSCs have been isolated from many different tissue sources, but little is known about how paracrine factor secretion varies between different MSC populations. We evaluated paracrine factor expression patterns in MSCs isolated from adipose tissue (ASCs), bone marrow (BMSCs), and dermal tissues [dermal sheath cells (DSCs) and dermal papilla cells (DPCs)]. Specifically, mRNA expression analysis identified insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor-D (VEGF-D), and interleukin-8 (IL-8) to be expressed at higher levels in ASCs compared with other MSC populations whereas VEGF-A, angiogenin, basic fibroblast growth factor (bFGF), and nerve growth factor (NGF) were expressed at comparable levels among the MSC populations examined. Analysis of conditioned media (CM) protein confirmed the comparable level of angiogenin and VEGF-A secretion in all MSC populations and showed that DSCs and DPCs produced significantly higher concentrations of leptin. Functional assays examining in vitro angiogenic paracrine activity showed that incubation of endothelial cells in ASC(CM) resulted in increased tubulogenic efficiency compared with that observed in DPC(CM). Using neutralizing antibodies we concluded that VEGF-A and VEGF-D were 2 of the major growth factors secreted by ASCs that supported endothelial tubulogenesis. The variation in paracrine factors of different MSC populations contributes to different levels of angiogenic activity and ASCs maybe preferred over other MSC populations for augmenting therapeutic approaches dependent upon angiogenesis.
Figures


References
-
- Johnston IA. Capillarisation, oxygen diffusion distances and mitochondrial content of carp muscles following acclimation to summer and winter temperatures. Cell Tissue Res. 1982;222:325–337. - PubMed
-
- Haynesworth SE. Goshima J. Goldberg VM. Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. - PubMed
-
- Yoo JU. Barthel TS. Nishimura K. Solchaga L. Caplan AI. Goldberg VM. Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–1757. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

