Pharmacological basis and clinical evidence of dabigatran therapy
- PMID: 22189016
- PMCID: PMC3262761
- DOI: 10.1186/1756-8722-4-53
Pharmacological basis and clinical evidence of dabigatran therapy
Abstract
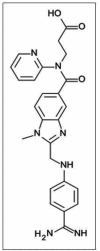
Dabigatran is an emerging oral anticoagulant which is a direct inhibitor of thrombin activity. It has been approved in the European Union and the United States of America for the prevention of thrombosis after major orthopedic surgery. It has also been approved by the American Food and Drug Administration and the European Medicines Agency for the prevention of stroke in chronic atrial fibrillation. Dabigatran provides a stable anticoagulation effect without any need to perform periodical laboratory controls. Of note, there is a growing amount of clinical evidence which shows its safety and efficacy. For these reasons, dabigatran may suppose a revolution in oral anticoagulation. However, two important limitations remain. First, it is contraindicated in patients with end-stage renal disease. Second, there is no evidence of the prevention of thrombosis in mechanical heart valves.
Figures
References
-
- Wienen W, Stassen JM, Priepke H. In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost. 2007;98:155–162. - PubMed
-
- Eriksson BI, Dahl OE, Ahnfelt L, Kälebo P, Stangier J, Nehmiz G, Hermansson K, Kohlbrenner V. Dose escalating safety study of a new oral direct thrombin inhibitor, dabigatran etexilate, in patients undergoing total hip replacement: BISTRO I. J Thromb Haemost. 2004;2:1573–1580. doi: 10.1111/j.1538-7836.2004.00890.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical