TLR3 signaling is either protective or pathogenic for the development of Theiler's virus-induced demyelinating disease depending on the time of viral infection
- PMID: 22189096
- PMCID: PMC3293102
- DOI: 10.1186/1742-2094-8-178
TLR3 signaling is either protective or pathogenic for the development of Theiler's virus-induced demyelinating disease depending on the time of viral infection
Abstract
Background: We have previously shown that toll-like receptor 3 (TLR3)-mediated signaling plays an important role in the induction of innate cytokine responses to Theiler's murine encephalomyelitis virus (TMEV) infection. In addition, cytokine levels produced after TMEV infection are significantly higher in the glial cells of susceptible SJL mice compared to those of resistant C57BL/6 mice. However, it is not known whether TLR3-mediated signaling plays a protective or pathogenic role in the development of demyelinating disease.
Methods: SJL/J and B6;129S-Tlr3tm1Flv/J (TLR3KO-B6) mice, and TLR3KO-SJL mice that TLR3KO-B6 mice were backcrossed to SJL/J mice for 6 generations were infected with Theiler's murine encephalomyelitis virus (2 × 105 PFU) with or without treatment with 50 μg of poly IC. Cytokine production and immune responses in the CNS and periphery of infected mice were analyzed.
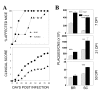
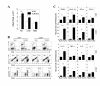
Results: We investigated the role of TLR3-mediated signaling in the protection and pathogenesis of TMEV-induced demyelinating disease. TLR3KO-B6 mice did not develop demyelinating disease although they displayed elevated viral loads in the CNS. However, TLR3KO-SJL mice displayed increased viral loads and cellular infiltration in the CNS, accompanied by exacerbated development of demyelinating disease, compared to the normal littermate mice. Late, but not early, anti-viral CD4+ and CD8+ T cell responses in the CNS were compromised in TLR3KO-SJL mice. However, activation of TLR3 with poly IC prior to viral infection also exacerbated disease development, whereas such activation after viral infection restrained disease development. Activation of TLR3 signaling prior to viral infection hindered the induction of protective IFN-γ-producing CD4+ and CD8+ T cell populations. In contrast, activation of these signals after viral infection improved the induction of IFN-γ-producing CD4+ and CD8+ T cells. In addition, poly IC-pretreated mice displayed elevated PDL-1 and regulatory FoxP3+ CD4+ T cells in the CNS, while poly IC-post-treated mice expressed reduced levels of PDL-1 and FoxP3+ CD4+ T cells.
Conclusions: These results suggest that TLR3-mediated signaling during viral infection protects against demyelinating disease by reducing the viral load and modulating immune responses. In contrast, premature activation of TLR3 signal transduction prior to viral infection leads to pathogenesis via over-activation of the pathogenic immune response.
Figures





References
-
- Akira S, Hoshino K, Kaisho T. The role of Toll-like receptors and MyD88 in innate immune responses. J Endotoxin Res. 2000;6:383–387. - PubMed
-
- Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280:5571–5580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

