In vitro culture medium influences the vaccine efficacy of Mycobacterium bovis BCG
- PMID: 22189700
- PMCID: PMC3269512
- DOI: 10.1016/j.vaccine.2011.12.044
In vitro culture medium influences the vaccine efficacy of Mycobacterium bovis BCG
Abstract
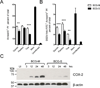
The varied rates of protection induced by Mycobacterium bovis BCG vaccine against tuberculosis has been attributed to many factors such as genetic variability among BCG strains, rapid clearance of BCG in some populations, and different levels of previous exposure of vaccinated populations to environmental mycobacteria. However, the methods and conditions employed to prepare this vaccine for human usage by various manufacturers have not been investigated as potential factors contributing to the variation in vaccine efficacy. A review of the literature indicates discrepancies between the approach for growing BCG vaccine in the laboratory to assess immune responses and protective ability in animal models, and that employed for production of the vaccine for administration to humans. One of the major differences is in the growth medium used for routine propagation in the laboratory and the one used for bulk vaccine production by manufacturers. Here we compared the immunogenicity of the BCG vaccine grown in Middlebrook 7H9 medium, the most commonly used medium in laboratory studies, against that grown in Sauton medium, which is used for growing BCG by most manufacturers. Our results showed clear differences in the behavior of BCG grown in these different culture media. Compared to BCG grown in Middlebrook 7H9 medium, BCG grown in Sauton media was more persistent inside macrophages, more effective at inhibiting apoptosis of infected cells, induced stronger inflammatory responses and stimulated less effective immunity against aerosol challenge with a virulent Mtb strain. These findings suggested that the growth medium used for producing BCG vaccine is an important factor that deserves increased scrutiny in ongoing efforts to produce more consistently effective vaccines against Mtb.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



References
-
- Oettinger T, Jorgensen M, Ladefoged A, Haslov K, Andersen P. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuberc Lung Dis. 1999;79(4):243–250. - PubMed
-
- Ritz N, Curtis N. Mapping the global use of different BCG vaccine strains. Tuberculosis (Edinb) 2009 July;89(4):248–251. - PubMed
-
- Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995 November;346(8986):1339–1345. - PubMed
-
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. J Am Med Assoc. 1994 March;271(9):698–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

