Cardiomyocyte death: mechanisms and translational implications
- PMID: 22190003
- PMCID: PMC3252742
- DOI: 10.1038/cddis.2011.130
Cardiomyocyte death: mechanisms and translational implications
Abstract
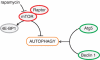
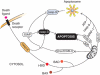
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide. Although treatments have improved, development of novel therapies for patients with CVD remains a major research goal. Apoptosis, necrosis, and autophagy occur in cardiac myocytes, and both gradual and acute cell death are hallmarks of cardiac pathology, including heart failure, myocardial infarction, and ischemia/reperfusion. Pharmacological and genetic inhibition of autophagy, apoptosis, or necrosis diminishes infarct size and improves cardiac function in these disorders. Here, we review recent progress in the fields of autophagy, apoptosis, and necrosis. In addition, we highlight the involvement of these mechanisms in cardiac pathology and discuss potential translational implications.
Figures
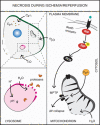
References
-
- Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. - PubMed
-
- Marambio P, Toro B, Sanhueza C, Troncoso R, Parra V, Verdejo H, et al. Glucose deprivation causes oxidative stress and stimulates aggresome formation and autophagy in cultured cardiac myocytes. Biochim Biophys Acta. 2010;1802:509–518. - PubMed
-
- Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

