Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity
- PMID: 22190239
- PMCID: PMC4517192
- DOI: 10.1126/scitranslmed.3003084
Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity
Abstract
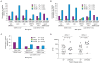
The rapid spread of dengue is a worldwide public health problem. In two clinical studies of dengue in Managua, Nicaragua, we observed an abrupt increase in disease severity across several epidemic seasons of dengue virus serotype 2 (DENV-2) transmission. Waning DENV-1 immunity appeared to increase the risk of severe disease in subsequent DENV-2 infections after a period of cross-protection. The increase in severity coincided with replacement of the Asian/American DENV-2 NI-1 clade with a new virus clade, NI-2B. In vitro analyses of viral isolates from the two clades and analysis of viremia in patient blood samples support the emergence of a fitter virus in later, relative to earlier, epidemic seasons. In addition, the NI-1 clade of viruses was more virulent specifically in children who were immune to DENV-1, whereas DENV-3 immunity was associated with more severe disease among NI-2B infections. Our data demonstrate that the complex interaction between viral genetics and population dynamics of serotype-specific immunity contributes to the risk of severe dengue disease. Furthermore, this work provides insights into viral evolution and the interaction between viral and immunological determinants of viral fitness and virulence.
Conflict of interest statement
Figures


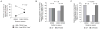

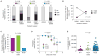
References
-
- World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2. World Health Organization; Geneva: 1997.
-
- Halstead SB. Dengue. Lancet. 2007;370:1644–1652. - PubMed
-
- Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halstead SB. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–669. - PubMed
-
- Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, Aye KM, Aaskov J. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–572. - PubMed
-
- Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. - PubMed
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

