The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations
- PMID: 22190486
- PMCID: PMC3258636
- DOI: 10.1073/pnas.1118857109
The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations
Abstract
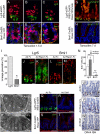
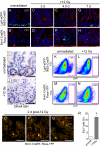
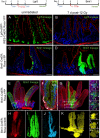
The small intestine epithelium undergoes rapid and continuous regeneration supported by crypt intestinal stem cells (ISCs). Bmi1 and Lgr5 have been independently identified to mark long-lived multipotent ISCs by lineage tracing in mice; however, the functional distinctions between these two populations remain undefined. Here, we demonstrate that Bmi1 and Lgr5 mark two functionally distinct ISCs in vivo. Lgr5 marks mitotically active ISCs that exhibit exquisite sensitivity to canonical Wnt modulation, contribute robustly to homeostatic regeneration, and are quantitatively ablated by irradiation. In contrast, Bmi1 marks quiescent ISCs that are insensitive to Wnt perturbations, contribute weakly to homeostatic regeneration, and are resistant to high-dose radiation injury. After irradiation, however, the normally quiescent Bmi1(+) ISCs dramatically proliferate to clonally repopulate multiple contiguous crypts and villi. Clonogenic culture of isolated single Bmi1(+) ISCs yields long-lived self-renewing spheroids of intestinal epithelium that produce Lgr5-expressing cells, thereby establishing a lineage relationship between these two populations in vitro. Taken together, these data provide direct evidence that Bmi1 marks quiescent, injury-inducible reserve ISCs that exhibit striking functional distinctions from Lgr5(+) ISCs and support a model whereby distinct ISC populations facilitate homeostatic vs. injury-induced regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. - PubMed
-
- Snippert HJ, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. - PubMed
-
- Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145:851–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1U01DK085527-01/DK/NIDDK NIH HHS/United States
- T32 CA009302/CA/NCI NIH HHS/United States
- 1R01DK085720-01/DK/NIDDK NIH HHS/United States
- R01 DK085720/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- K08 DK096048/DK/NIDDK NIH HHS/United States
- U24 DK085532/DK/NIDDK NIH HHS/United States
- R01 DK069989/DK/NIDDK NIH HHS/United States
- R37 HD030701/HD/NICHD NIH HHS/United States
- U01 DK085532/DK/NIDDK NIH HHS/United States
- R01 GM021168/GM/NIGMS NIH HHS/United States
- 1R01 DK069989-01/DK/NIDDK NIH HHS/United States
- T32 AI007328/AI/NIAID NIH HHS/United States
- U01 DK085527/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

