Characterization and adaptive optical correction of aberrations during in vivo imaging in the mouse cortex
- PMID: 22190489
- PMCID: PMC3252919
- DOI: 10.1073/pnas.1109202108
Characterization and adaptive optical correction of aberrations during in vivo imaging in the mouse cortex
Abstract
The signal and resolution during in vivo imaging of the mouse brain is limited by sample-induced optical aberrations. We find that, although the optical aberrations can vary across the sample and increase in magnitude with depth, they remain stable for hours. As a result, two-photon adaptive optics can recover diffraction-limited performance to depths of 450 μm and improve imaging quality over fields of view of hundreds of microns. Adaptive optical correction yielded fivefold signal enhancement for small neuronal structures and a threefold increase in axial resolution. The corrections allowed us to detect smaller neuronal structures at greater contrast and also improve the signal-to-noise ratio during functional Ca(2+) imaging in single neurons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

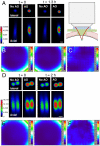
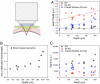
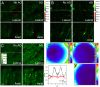
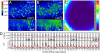
References
-
- Kerr JND, Denk W. Imaging in vivo: Watching the brain in action. Nat Rev Neurosci. 2008;9:195–205. - PubMed
-
- Schwertner M, Booth MJ, Neil MAA, Wilson T. Measurement of specimen-induced aberrations of biological samples using phase stepping interferometry. J Microsc. 2004;213:11–19. - PubMed
-
- Schwertner M, Booth MJ, Wilson T. Characterizing specimen induced aberrations for high NA adaptive optical microscopy. Opt Express. 2004;12:6540–6552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

