Wnt/β-catenin signaling directly regulates Foxj1 expression and ciliogenesis in zebrafish Kupffer's vesicle
- PMID: 22190638
- PMCID: PMC4074261
- DOI: 10.1242/dev.071746
Wnt/β-catenin signaling directly regulates Foxj1 expression and ciliogenesis in zebrafish Kupffer's vesicle
Abstract
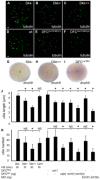
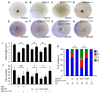
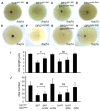
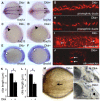
Cilia are essential for normal development. The composition and assembly of cilia has been well characterized, but the signaling and transcriptional pathways that govern ciliogenesis remain poorly studied. Here, we report that Wnt/β-catenin signaling directly regulates ciliogenic transcription factor foxj1a expression and ciliogenesis in zebrafish Kupffer's vesicle (KV). We show that Wnt signaling acts temporally and KV cell-autonomously to control left-right (LR) axis determination and ciliogenesis. Specifically, reduction of Wnt signaling leads to a disruption of LR patterning, shorter and fewer cilia, a loss of cilia motility and a downregulation of foxj1a expression. However, these phenotypes can be rescued by KV-targeted overexpression of foxj1a. In comparison to the FGF pathway that has been previously implicated in the control of ciliogenesis, our epistatic studies suggest a more downstream function of Wnt signaling in the regulation of foxj1a expression and ciliogenesis in KV. Importantly, enhancer analysis reveals that KV-specific expression of foxj1a requires the presence of putative Lef1/Tcf binding sites, indicating that Wnt signaling activates foxj1a transcription directly. We also find that impaired Wnt signaling leads to kidney cysts and otolith disorganization, which can be attributed to a loss of foxj1 expression and disrupted ciliogenesis in the developing pronephric ducts and otic vesicles. Together, our data reveal a novel role of Wnt/β-catenin signaling upstream of ciliogenesis, which might be a general developmental mechanism beyond KV. Moreover, our results also prompt a hypothesis that certain developmental effects of the Wnt/β-catenin pathway are due to the activation of Foxj1 and cilia formation.
Figures


References
-
- Afzelius B. A. (1976). A human syndrome caused by immotile cilia. Science 193, 317–319 - PubMed
-
- Alexander J., Rothenberg M., Henry G. L., Stainier D. Y. (1999). Casanova plays an early and essential role in endoderm formation in zebrafish. Dev. Biol. 215, 343–357 - PubMed
-
- Amack J. D., Yost H. J. (2004). The T box transcription factor no tail in ciliated cells controls zebrafish left-right asymmetry. Curr. Biol. 14, 685–690 - PubMed
-
- Badano J. L., Mitsuma N., Beales P. L., Katsanis N. (2006). The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7, 125–148 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

