Increased macrophage migration into adipose tissue in obese mice
- PMID: 22190646
- PMCID: PMC3266418
- DOI: 10.2337/db11-0860
Increased macrophage migration into adipose tissue in obese mice
Abstract
Macrophage-mediated inflammation is a key component of insulin resistance; however, the initial events of monocyte migration to become tissue macrophages remain poorly understood. We report a new method to quantitate in vivo macrophage tracking (i.e., blood monocytes from donor mice) labeled ex vivo with fluorescent PKH26 dye and injected into recipient mice. Labeled monocytes appear as adipose, liver, and splenic macrophages, peaking in 1-2 days. When CCR2 KO monocytes are injected into wild-type (WT) recipients, or WT monocytes given to MCP-1 KO recipients, adipose tissue macrophage (ATM) accumulation is reduced by ~40%, whereas hepatic macrophage content is decreased by ~80%. Using WT donor cells, ATM accumulation is several-fold greater in obese recipient mice compared with lean mice, regardless of the source of donor monocytes. After their appearance in adipose tissue, ATMs progressively polarize from the M2- to the M1-like state in obesity. In summary, the CCR2/MCP-1 system is a contributory factor to monocyte migration into adipose tissue and is the dominant signal controlling the appearance of recruited macrophages in the liver. Monocytes from obese mice are not programmed to become inflammatory ATMs but rather the increased proinflammatory ATM accumulation in obesity is in response to tissue signals.
Figures



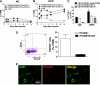
References
-
- Olefsky JM, Courtney CH. Type 2 diabetes mellitus: etiology, pathogenesis, and natural history. In DeGroot Textbook of Endocrinology. 5th ed. Philadelphia, W.B. Saunders and Company, 2005, p. 1093–1117
-
- Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr 2005;25:391–406 - PubMed
-
- Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–887 - PubMed
-
- Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome. Circulation 2003;108:1541–1545 - PubMed
-
- Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol 1997;146:214–222 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

