Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia
- PMID: 22190737
- PMCID: PMC3279387
- DOI: 10.1091/mbc.E11-09-0791
Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia
Abstract
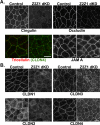
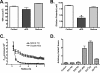
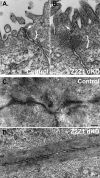
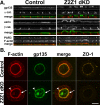
The structure and function of both adherens (AJ) and tight (TJ) junctions are dependent on the cortical actin cytoskeleton. The zonula occludens (ZO)-1 and -2 proteins have context-dependent interactions with both junction types and bind directly to F-actin and other cytoskeletal proteins, suggesting ZO-1 and -2 might regulate cytoskeletal activity at cell junctions. To address this hypothesis, we generated stable Madin-Darby canine kidney cell lines depleted of both ZO-1 and -2. Both paracellular permeability and the localization of TJ proteins are disrupted in ZO-1/-2-depleted cells. In addition, immunocytochemistry and electron microscopy revealed a significant expansion of the perijunctional actomyosin ring associated with the AJ. These structural changes are accompanied by a recruitment of 1-phosphomyosin light chain and Rho kinase 1, contraction of the actomyosin ring, and expansion of the apical domain. Despite these changes in the apical cytoskeleton, there are no detectable changes in cell polarity, localization of AJ proteins, or the organization of the basal and lateral actin cytoskeleton. We conclude that ZO proteins are required not only for TJ assembly but also for regulating the organization and functional activity of the apical cytoskeleton, particularly the perijunctional actomyosin ring, and we speculate that these activities are relevant both to cellular organization and epithelial morphogenesis.
Figures






References
-
- Ando-Akatsuka Y, Yonemura S, Itoh M, Furuse M, Tsukita S. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J Cell Physiol. 1999;179:115–125. - PubMed
-
- Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

