Androgen Receptor Regulates Transcription of the ZEB1 Transcription Factor
- PMID: 22190929
- PMCID: PMC3235469
- DOI: 10.1155/2011/903918
Androgen Receptor Regulates Transcription of the ZEB1 Transcription Factor
Abstract
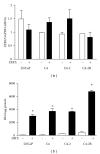
The zinc finger E-box binding protein 1 (ZEB1) transcription factor belongs to a two-member family of zinc-finger homeodomain proteins involved in physiological and pathological events mostly relating to cell migration and epithelial to mesenchymal transitions (EMTs). ZEB1 (also known as δEF1, zfhx1a, TCF8, and Zfhep) plays a key role in regulating such diverse processes as T-cell development, skeletal patterning, reproduction, and cancer cell metastasis. However, the factors that regulate its expression and consequently the signaling pathways in which ZEB1 participates are poorly defined. Because it is induced by estrogen and progesterone and is high in prostate cancer, we investigated whether tcf8, which encodes ZEB1, is regulated by androgen. Data herein demonstrate that tcf8 is induced by dihydrotestosterone (DHT) in the human PC-3/AR prostate cancer cell line and that this induction is mediated by two androgen response elements (AREs). These results demonstrate that ZEB1 is an intermediary in androgen signaling pathways.
Figures


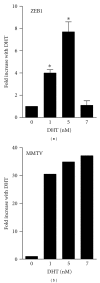
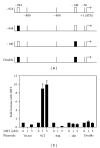
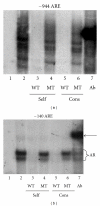
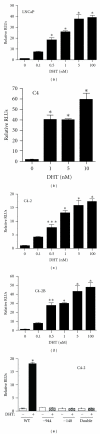
References
-
- Dillner NB, Sanders MM. Transcriptional activation by the zinc-finger homeodomain protein δEF1 in estrogen signaling cascades. DNA and Cell Biology. 2004;23(1):25–34. - PubMed
-
- Lazarova DL, Bordonaro M, Sartorelli AC. Transcriptional regulation of the vitamin D3 receptor gene by ZEB. Cell Growth and Differentiation. 2001;12(6):319–326. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

