Role of oxidative stress in transformation induced by metal mixture
- PMID: 22191014
- PMCID: PMC3236433
- DOI: 10.1155/2011/935160
Role of oxidative stress in transformation induced by metal mixture
Abstract
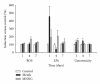
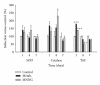
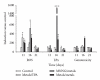
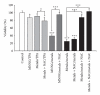
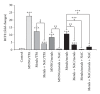
Metals are ubiquitous pollutants present as mixtures. In particular, mixture of arsenic-cadmium-lead is among the leading toxic agents detected in the environment. These metals have carcinogenic and cell-transforming potential. In this study, we used a two step cell transformation model, to determine the role of oxidative stress in transformation induced by a mixture of arsenic-cadmium-lead. Oxidative damage and antioxidant response were determined. Metal mixture treatment induces the increase of damage markers and the antioxidant response. Loss of cell viability and increased transforming potential were observed during the promotion phase. This finding correlated significantly with generation of reactive oxygen species. Cotreatment with N-acetyl-cysteine induces effect on the transforming capacity; while a diminution was found in initiation, in promotion phase a total block of the transforming capacity was observed. Our results suggest that oxidative stress generated by metal mixture plays an important role only in promotion phase promoting transforming capacity.
Figures




References
-
- Carrizales L, Razo I, Téllez-Hernández JI, et al. Exposure to arsenic and lead of children living near a copper-smelter in San Luis Potosi, Mexico: importance of soil contamination for exposure of children. Environmental Research. 2006;101(1):1–10. - PubMed
-
- Bae DS, Gennings C, Carter WH, Jr., Yang RSH, Campain JA. Toxicological interactions among arsenic, cadmium, chromium, and lead in human keratinocytes. Toxicological Sciences. 2001;63(1):132–142. - PubMed
-
- Wang G, Fowler BA. Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic. Toxicology and Applied Pharmacology. 2008;233(1):92–99. - PubMed
-
- ATSDR. Priority List of Hazardous Substances. Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services; 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

