Ionic residues of human serum transferrin affect binding to the transferrin receptor and iron release
- PMID: 22191507
- PMCID: PMC3267578
- DOI: 10.1021/bi201661g
Ionic residues of human serum transferrin affect binding to the transferrin receptor and iron release
Abstract
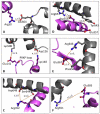
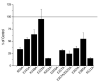
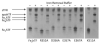
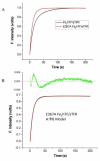
Efficient delivery of iron is critically dependent on the binding of diferric human serum transferrin (hTF) to its specific receptor (TFR) on the surface of actively dividing cells. Internalization of the complex into an endosome precedes iron removal. The return of hTF to the blood to continue the iron delivery cycle relies on the maintenance of the interaction between apohTF and the TFR after exposure to endosomal pH (≤6.0). Identification of the specific residues accounting for the pH-sensitive nanomolar affinity with which hTF binds to TFR throughout the cycle is important to fully understand the iron delivery process. Alanine substitution of 11 charged hTF residues identified by available structures and modeling studies allowed evaluation of the role of each in (1) binding of hTF to the TFR and (2) TFR-mediated iron release. Six hTF mutants (R50A, R352A, D356A, E357A, E367A, and K511A) competed poorly with biotinylated diferric hTF for binding to TFR. In particular, we show that Asp356 in the C-lobe of hTF is essential to the formation of a stable hTF-TFR complex: mutation of Asp356 in the monoferric C-lobe hTF background prevented the formation of the stoichiometric 2:2 (hTF:TFR monomer) complex. Moreover, mutation of three residues (Asp356, Glu367, and Lys511), whether in the diferric or monoferric C-lobe hTF, significantly affected iron release when in complex with the TFR. Thus, mutagenesis of charged hTF residues has allowed identification of a number of residues that are critical to formation of and release of iron from the hTF-TFR complex.
Figures
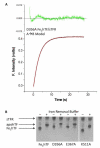
References
-
- Zak O, Aisen P. Nonrandom distribution of iron in circulating human transferrin. Blood. 1986;68:157–161. - PubMed
-
- Lawrence CM, Ray S, Babyonyshev M, Galluser R, Borhani DW, Harrison SC. Crystal structure of the ectodomain of human transferrin receptor. Science. 1999;286:779–782. - PubMed
-
- Mason AB, Byrne SL, Everse SJ, Roberts SE, Chasteen ND, Smith VC, Macgillivray RT, Kandemir B, Bou-Abdallah F. A loop in the N-lobe of human serum transferrin is critical for binding to the transferrin receptor as revealed by mutagenesis, isothermal titration calorimetry, and epitope mapping. J. Mol. Recognit. 2009;22:521–529. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

