Rapid S-nitrosylation of actin by NO-generating donors and in inflammatory pain model mice
- PMID: 22192148
- PMCID: PMC3295738
- DOI: 10.1186/1744-8069-7-101
Rapid S-nitrosylation of actin by NO-generating donors and in inflammatory pain model mice
Abstract
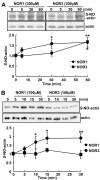
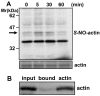
Background: S-Nitrosylation, the reversible post-translational modification of reactive cysteine residues in proteins, has emerged as an important mechanism by which NO acts as a signaling molecule. We recently demonstrated that actin is a major S-nitrosylated protein in the spinal cord and suggested that NO directly attenuates dopamine release from PC12 cells by causing the breakdown of F-actin. However, the occurrence of S-nitrosylation of actin remained unclarified in animal pain model. Kinetic analysis of S-nitrosylation of actin in the present study was made by using NO-generating donors. The biotin-switch assay and purification on streptavidin-agarose were employed for identification of S-nitrosylated actin.
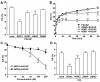
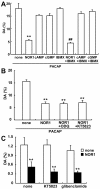
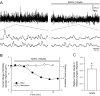
Results: Dopamine release from PC12 cells was markedly attenuated by NOR1 (t1/2 = 1.8 min) and much less by NOR3 (t1/2 = 30 min), but not by S-nitroso-glutathione, an endogenous NO donor. A membrane-permeable cGMP analogue could not substitute for NOR1 as a suppressor nor could inhibitors of soluble guanylate cyclase and cGMP-dependent protein kinase attenuate the suppression. S-Nitrosylated actin was detected by the biotin-switch assay at 5 min after the addition of NOR1. Consistent with the kinetic analysis, actin in the spinal cord was rapidly and maximally S-nitrosylated in an inflammatory pain model at 5 min after the injection of 2% formalin into the hind paws. In vivo patch-clamp recordings of the spinal dorsal horn, NOR3 showed an inhibitory action on inhibitory synaptic transmission in interneurons of the substantia gelatinosa.
Conclusions: The present study demonstrates that rapid S-nitrosylation of actin occurred in vitro in the presence of exogenous NO-generating donors and in vivo in inflammatory pain model mice. Our data suggest that, in addition to the well-known cGMP-dependent protein kinase pathway, S-nitrosylation is involved in pain transmission via disinhibition of inhibitory neurons.
Figures


Similar articles
-
Involvement of S-nitrosylation of actin in inhibition of neurotransmitter release by nitric oxide.Mol Pain. 2009 Sep 29;5:58. doi: 10.1186/1744-8069-5-58. Mol Pain. 2009. PMID: 19785772 Free PMC article.
-
Nitric oxide inhibits platelet adhesion to collagen through cGMP-dependent and independent mechanisms: the potential role for S-nitrosylation.Platelets. 2009 Nov;20(7):478-86. doi: 10.3109/09537100903159375. Platelets. 2009. PMID: 19852686
-
Nitric oxide blocks hKv1.5 channels by S-nitrosylation and by a cyclic GMP-dependent mechanism.Cardiovasc Res. 2006 Oct 1;72(1):80-9. doi: 10.1016/j.cardiores.2006.06.021. Epub 2006 Jun 27. Cardiovasc Res. 2006. PMID: 16876149
-
Nitric oxide inhibits nociceptive transmission by differentially regulating glutamate and glycine release to spinal dorsal horn neurons.J Biol Chem. 2011 Sep 23;286(38):33190-202. doi: 10.1074/jbc.M111.270967. Epub 2011 Aug 3. J Biol Chem. 2011. PMID: 21813646 Free PMC article.
-
Nitrosative Stress in the Nervous System: Guidelines for Designing Experimental Strategies to Study Protein S-Nitrosylation.Neurochem Res. 2016 Mar;41(3):510-4. doi: 10.1007/s11064-015-1640-z. Epub 2015 Jun 29. Neurochem Res. 2016. PMID: 26118537 Free PMC article. Review.
Cited by
-
Crucial involvement of actin filaments in celecoxib and morphine analgesia in a model of inflammatory pain.J Pain Res. 2012;5:535-45. doi: 10.2147/JPR.S36870. Epub 2012 Nov 9. J Pain Res. 2012. PMID: 23166451 Free PMC article.
-
Protein-S-nitrosylation of human cytomegalovirus pp65 reduces its ability to undermine cGAS.J Virol. 2025 May 20;99(5):e0048125. doi: 10.1128/jvi.00481-25. Epub 2025 Apr 17. J Virol. 2025. PMID: 40243337 Free PMC article.
-
Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain.Neural Regen Res. 2023 May;18(5):996-1003. doi: 10.4103/1673-5374.355748. Neural Regen Res. 2023. PMID: 36254980 Free PMC article. Review.
-
Nitric oxide and carbon monoxide antagonize TGF-β through ligand-independent internalization of TβR1/ALK5.Am J Physiol Renal Physiol. 2014 Sep 15;307(6):F727-35. doi: 10.1152/ajprenal.00353.2014. Epub 2014 Aug 6. Am J Physiol Renal Physiol. 2014. PMID: 25100282 Free PMC article.
-
Microglial activation induces nitric oxide signalling and alters protein S-nitrosylation patterns in extracellular vesicles.J Extracell Vesicles. 2024 Jun;13(6):e12455. doi: 10.1002/jev2.12455. J Extracell Vesicles. 2024. PMID: 38887871 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

