The ins and outs of Mycobacterium tuberculosis protein export
- PMID: 22192870
- PMCID: PMC3288827
- DOI: 10.1016/j.tube.2011.11.005
The ins and outs of Mycobacterium tuberculosis protein export
Abstract
Mycobacterium tuberculosis is an important pathogen that infects approximately one-third of the world's population and kills almost two million people annually. An important aspect of M. tuberculosis physiology and pathogenesis is its ability to export proteins into and across the thick mycobacterial cell envelope, where they are ideally positioned to interact with the host. In addition to the specific proteins that are exported by M. tuberculosis, the systems through which these proteins are exported represent potential targets for future drug development. M. tuberculosis possesses two well-known and conserved export systems: the housekeeping Sec pathway and the Tat pathway. In addition, M. tuberculosis possesses specialized export systems including the accessory SecA2 pathway and five ESX pathways. Here we review the current understanding of each of these export systems, with a focus on M. tuberculosis, and discuss the contribution of each system to disease and physiology.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
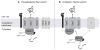
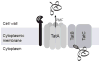

References
-
- WHO/HTM/TB2010.7 Pn. WHO Report 2010: Global Tuberculosis Control. Geneva, Switzerland: WHO Press; 2010.
-
- Moir DT, Di M, Wong E, Moore RA, Schweizer HP, Woods DE, Bowlin TL. Development and application of a cellular, gain-of-signal, bioluminescent reporter screen for inhibitors of type II secretion in Pseudomonas aeruginosa and Burkholderia pseudomallei. J Biomol Screen. 2011;16:694–705. doi: 10.1177/1087057111408605. - DOI - PMC - PubMed
-
- Paschos A, den Hartigh A, Smith MA, Atluri VL, Sivanesan D, Tsolis RM, Baron C. An in vivo high-throughput screening approach targeting the type IV secretion system component VirB8 identified inhibitors of Brucella abortus 2308 proliferation. Infect Immun. 2011;79:1033–1043. doi: 10.1128/IAI.00993-10. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

