Lessons from yeast for clathrin-mediated endocytosis
- PMID: 22193158
- PMCID: PMC5590828
- DOI: 10.1038/ncb2403
Lessons from yeast for clathrin-mediated endocytosis
Abstract
Clathrin-mediated endocytosis (CME) is the major pathway for internalization of membrane proteins from the cell surface. Half a century of studies have uncovered tremendous insights into how a clathrin-coated vesicle is formed. More recently, the advent of live-cell imaging has provided a dynamic view of this process. As CME is highly conserved from yeast to humans, budding yeast provides an evolutionary template for this process and has been a valuable system for dissecting the underlying molecular mechanisms. In this review we trace the formation of a clathrin-coated vesicle from initiation to uncoating, focusing on key findings from the yeast system.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

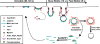
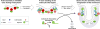
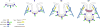
References
-
- Crowther RA, Finch JT, Pearse BM. On the structure of coated vesicles. J Mol Biol. 1976;103:785–798. - PubMed
-
- Pearse BM. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975;97:93–98. - PubMed
-
- Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

