The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults
- PMID: 22193671
- PMCID: PMC3893686
- DOI: 10.1176/appi.ajp.2011.10111704
The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults
Abstract
Objective: Research on suicide prevention and interventions requires a standard method for assessing both suicidal ideation and behavior to identify those at risk and to track treatment response. The Columbia-Suicide Severity Rating Scale (C-SSRS) was designed to quantify the severity of suicidal ideation and behavior. The authors examined the psychometric properties of the scale.
Method: The C-SSRS's validity relative to other measures of suicidal ideation and behavior and the internal consistency of its intensity of ideation subscale were analyzed in three multisite studies: a treatment study of adolescent suicide attempters (N=124); a medication efficacy trial with depressed adolescents (N=312); and a study of adults presenting to an emergency department for psychiatric reasons (N=237).
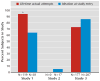
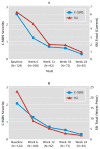
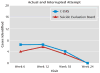
Results: The C-SSRS demonstrated good convergent and divergent validity with other multi-informant suicidal ideation and behavior scales and had high sensitivity and specificity for suicidal behavior classifications compared with another behavior scale and an independent suicide evaluation board. Both the ideation and behavior subscales were sensitive to change over time. The intensity of ideation subscale demonstrated moderate to strong internal consistency. In the adolescent suicide attempters study, worst-point lifetime suicidal ideation on the C-SSRS predicted suicide attempts during the study, whereas the Scale for Suicide Ideation did not. Participants with the two highest levels of ideation severity (intent or intent with plan) at baseline had higher odds for attempting suicide during the study.
Conclusions: These findings suggest that the C-SSRS is suitable for assessment of suicidal ideation and behavior in clinical and research settings.
Figures

Comment in
-
Objective assessment of suicide risk: significant improvements in assessment, classification, and prediction.Am J Psychiatry. 2011 Dec;168(12):1233-4. doi: 10.1176/appi.ajp.2011.11091362. Am J Psychiatry. 2011. PMID: 22193664 No abstract available.
-
Suicidal ideation and later suicide.Am J Psychiatry. 2012 Jun;169(6):662; author reply 663. doi: 10.1176/appi.ajp.2012.11111674. Am J Psychiatry. 2012. PMID: 22684596 No abstract available.
-
Initial validity and reliability data on the Columbia-Suicide Severity Rating Scale.Am J Psychiatry. 2012 Jun;169(6):662-3; author reply 663. doi: 10.1176/appi.ajp.2012.12010123. Am J Psychiatry. 2012. PMID: 22684597 No abstract available.
References
-
- National Strategy for Suicide Prevention: Goals and Objectives for Action. Rockville, Md: Public Health Service, US Department of Health and Human Services; 2001. - PubMed
-
- Crosby AE, Ortega L, Melanson C. Self-Directed Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 1.0. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Feb, 2011.
-
- Goldsmith SK, Pellmar TC, Kleinman AM, Bunney WE, editors. Reducing Suicide: A National Imperative. Washington, DC: National Academies Press; 2002. - PubMed
-
- Zimmerman M, Chelminski I, Posternak MA. Exclusion criteria used in antidepressant efficacy trials: consistency across studies and representativeness of samples included. J Nerv Ment Dis. 2004;192:87–94. - PubMed
-
- Zimmerman M, Chelminski I, Posternak MA. Generalizability of antidepressant efficacy trials: differences between depressed psychiatric outpatients who would or would not qualify for an efficacy trial. Am J Psychiatry. 2005;162:1370–1372. - PubMed
Publication types
MeSH terms
Grants and funding
- U10 MH066750/MH/NIMH NIH HHS/United States
- MH66769/MH/NIMH NIH HHS/United States
- U10 MH066762/MH/NIMH NIH HHS/United States
- U10 MH066775/MH/NIMH NIH HHS/United States
- P20 AA015630/AA/NIAAA NIH HHS/United States
- U10 MH066778/MH/NIMH NIH HHS/United States
- MH66762/MH/NIMH NIH HHS/United States
- MH66778/MH/NIMH NIH HHS/United States
- 5 P20AA015630/AA/NIAAA NIH HHS/United States
- MH66750/MH/NIMH NIH HHS/United States
- U10 MH066769/MH/NIMH NIH HHS/United States
- P50 MH090964/MH/NIMH NIH HHS/United States
- MH66775/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

