Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways
- PMID: 22193709
- PMCID: PMC4515451
- DOI: 10.1038/nri3131
Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways
Abstract
Pathogens specifically target both the caspase 8-dependent apoptotic cell death pathway and the necrotic cell death pathway that is dependent on receptor-interacting protein 1 (RIP1; also known as RIPK1) and RIP3 (also known as RIPK3). The fundamental co-regulation of these two cell death pathways emerged when the midgestational death of mice deficient in FAS-associated death domain protein (FADD) or caspase 8 was reversed by elimination of RIP1 or RIP3, indicating a far more entwined relationship than previously appreciated. Thus, mammals require caspase 8 activity during embryogenesis to suppress the kinases RIP1 and RIP3 as part of the dialogue between two distinct cell death processes that together fulfil reinforcing roles in the host defence against intracellular pathogens such as herpesviruses.
Figures
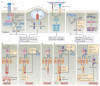

References
-
- Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annu. Rev. Biochem. 2000;69:217–245. - PubMed
-
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. - PubMed
-
- Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature Immunol. 2000;1:489–495. - PubMed
-
A seminal study that demonstrated that the kinase RIP1 is a mediator of necrosis induced by the death receptor FAS and that caspase 8 is a crucial negative regulator of the pathway.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

