The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV
- PMID: 22193774
- PMCID: PMC3378499
- DOI: 10.1097/QAI.0b013e3182432f27
The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV
Abstract
Background: The prevention of mother-to-child transmission (PMTCT) of HIV has been focused mainly on women who are HIV positive at their first antenatal visit, but there is uncertainty regarding the contribution to overall transmission from mothers who seroconvert after their first antenatal visit and before weaning.
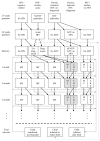
Method: A mathematical model was developed to simulate changes in mother-to-child transmission of HIV over time, in South Africa. The model allows for changes in infant feeding practices as infants age, temporal changes in the provision of antiretroviral prophylaxis and counseling on infant feeding, as well as temporal changes in maternal HIV prevalence and incidence.
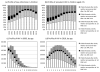
Results: The proportion of mother-to-child transmission (MTCT) from mothers who seroconverted after their first antenatal visit was 26% [95% confidence interval (CI): 22% to 30%] in 2008, or 15,000 of 57,000 infections. It is estimated that by 2014, total MTCT will reduce to 39,000 per annum, and transmission from mothers seroconverting after their first antenatal visit will reduce to 13,000 per annum, accounting for 34% (95% CI: 29% to 39%) of MTCT. If maternal HIV incidence during late pregnancy and breastfeeding were reduced by 50% after 2010, and HIV screening were repeated in late pregnancy and at 6-week immunization visits after 2010, the average annual number of MTCT cases over the 2010-2015 period would reduce by 28% (95% CI: 25% to 31%), from 39,000 to 28,000 per annum.
Conclusion: Maternal seroconversion during late pregnancy and breastfeeding contributes significantly to the pediatric HIV burden and needs greater attention in the planning of prevention of MTCT programs.
Figures
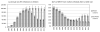
References
-
- Sperling RS, Shapiro DE, Coombs RW, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. N Engl J Med. 1996;335:1621–9. - PubMed
-
- Taha TE, Kumwenda NI, Hoover DR, et al. Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: a randomized controlled trial. JAMA. 2004;292:202–9. - PubMed
-
- Pillay K, Coutsoudis A, York D, et al. Cell-free virus in breast milk of HIV-1-seropositive women. J Acquir Immun Defic Syndr. 2000;24:330–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

