In vivo imaging of basement membrane movement: ECM patterning shapes Hydra polyps
- PMID: 22194305
- PMCID: PMC3244984
- DOI: 10.1242/jcs.087239
In vivo imaging of basement membrane movement: ECM patterning shapes Hydra polyps
Abstract
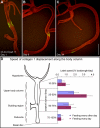
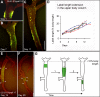
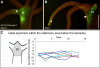
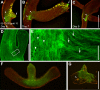
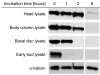
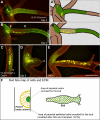
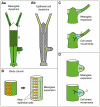
Growth and morphogenesis during embryonic development, asexual reproduction and regeneration require extensive remodeling of the extracellular matrix (ECM). We used the simple metazoan Hydra to examine the fate of ECM during tissue morphogenesis and asexual budding. In growing Hydra, epithelial cells constantly move towards the extremities of the animal and into outgrowing buds. It is not known, whether these tissue movements involve epithelial migration relative to the underlying matrix or whether cells and ECM are displaced as a composite structure. Furthermore, it is unclear, how the ECM is remodeled to adapt to the shape of developing buds and tentacles. To address these questions, we used a new in vivo labeling technique for Hydra collagen-1 and laminin, and tracked the fate of ECM in all body regions of the animal. Our results reveal that Hydra 'tissue movements' are largely displacements of epithelial cells together with associated ECM. By contrast, during the evagination of buds and tentacles, extensive movement of epithelial cells relative to the matrix is observed, together with local ECM remodeling. These findings provide new insights into the nature of growth and morphogenesis in epithelial tissues.
Figures



References
-
- Bertet C., Sulak L., Lecuit T. (2004). Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667-671 - PubMed
-
- Bosch T. C., David C. N. (1984). Growth regulation in Hydra: relationship between epithelial cell cycle length and growth rate. Dev. Biol. 104, 161-171 - PubMed
-
- Burnett A. L., Hausman R. E. (1969). Mesoglea of Hydra. 2. Possible Role in Morphogenesis. J. Exp. Zool. 171, 15-24 - PubMed
-
- Campbell R. D. (1967a). Tissue dynamics of steady state growth in Hydra littoralis. I. Patterns of cell division. Dev. Biol. 15, 487-502 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

