Heparanase induces signal transducer and activator of transcription (STAT) protein phosphorylation: preclinical and clinical significance in head and neck cancer
- PMID: 22194600
- PMCID: PMC3307274
- DOI: 10.1074/jbc.M111.271346
Heparanase induces signal transducer and activator of transcription (STAT) protein phosphorylation: preclinical and clinical significance in head and neck cancer
Abstract
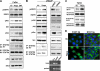
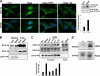
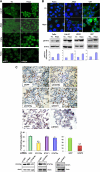
Activity of heparanase is implicated strongly in dissemination of metastatic tumor cells and cells of the immune system. In addition, heparanase enhances the phosphorylation of selected signaling molecules, including SRC and EGFR, in a manner that requires secretion but not enzymatic activity of heparanase and is mediated by its C-terminal domain. Clinically, heparanase staining is associated with larger tumors and increased EGFR phosphorylation in head and neck carcinoma. We hypothesized that signal transducer and activator of transcription (STAT) proteins mediate the protumorigenic function of heparanase downstream of the EGFR. We provide evidence that heparanase enhances the phosphorylation of STAT3 and STAT5b but not STAT5a. Moreover, enhanced proliferation of heparanase transfected cells was attenuated by STAT3 and STAT5b siRNA, but not STAT5a or STAT1 siRNA. Clinically, STAT3 phosphorylation was associated with head and neck cancer progression, EGFR phosphorylation, and heparanase expression and cellular localization. Notably, cytoplasmic rather than nuclear phospho-STAT3 correlated with increased tumor size (T-stage; p = 0.007), number of metastatic neck lymph nodes (p = 0.05), and reduced survival of patients (p = 0.04).
Figures


Similar articles
-
Heparanase augments epidermal growth factor receptor phosphorylation: correlation with head and neck tumor progression.Cancer Res. 2008 Dec 15;68(24):10077-85. doi: 10.1158/0008-5472.CAN-08-2910. Cancer Res. 2008. PMID: 19074873 Free PMC article.
-
STAT5A-mediated SOCS2 expression regulates Jak2 and STAT3 activity following c-Src inhibition in head and neck squamous carcinoma.Clin Cancer Res. 2012 Jan 1;18(1):127-39. doi: 10.1158/1078-0432.CCR-11-1889. Epub 2011 Nov 16. Clin Cancer Res. 2012. PMID: 22090359 Free PMC article.
-
Opposing Effects of Heparanase and Heparanase-2 in Head & Neck Cancer.Adv Exp Med Biol. 2020;1221:847-856. doi: 10.1007/978-3-030-34521-1_37. Adv Exp Med Biol. 2020. PMID: 32274741 Review.
-
Phosphorylation of STAT3 in head and neck cancer requires p38 MAPKinase, whereas phosphorylation of STAT1 occurs via a different signaling pathway.Anticancer Res. 2011 Nov;31(11):3819-25. Anticancer Res. 2011. PMID: 22110204
-
Proteoglycans in health and disease: new concepts for heparanase function in tumor progression and metastasis.FEBS J. 2010 Oct;277(19):3890-903. doi: 10.1111/j.1742-4658.2010.07799.x. Epub 2010 Aug 31. FEBS J. 2010. PMID: 20840586 Free PMC article. Review.
Cited by
-
Heparanase regulation of cancer, autophagy and inflammation: new mechanisms and targets for therapy.FEBS J. 2017 Jan;284(1):42-55. doi: 10.1111/febs.13932. Epub 2016 Nov 16. FEBS J. 2017. PMID: 27758044 Free PMC article. Review.
-
[Mechanisms of EGF regulation of COX-2 through the STAT5 signaling pathway in human lung adenocarcinoma A549 cells].Zhongguo Fei Ai Za Zhi. 2013 Apr;16(4):169-76. doi: 10.3779/j.issn.1009-3419.2013.04.01. Zhongguo Fei Ai Za Zhi. 2013. PMID: 23601296 Free PMC article. Chinese.
-
Role of a Novel Heparanase Inhibitor on the Balance between Apoptosis and Autophagy in U87 Human Glioblastoma Cells.Cells. 2023 Jul 19;12(14):1891. doi: 10.3390/cells12141891. Cells. 2023. PMID: 37508554 Free PMC article.
-
Characterization of the Goose CAPN3 Gene and its Expression Pattern in Muscle Tissues of Sichuan White Geese at Different Growth Stages.J Poult Sci. 2018;55(3):172-181. doi: 10.2141/jpsa.0170150. Epub 2018 Feb 25. J Poult Sci. 2018. PMID: 32055171 Free PMC article.
-
The multifaceted role of STAT3 pathway and its implication as a potential therapeutic target in oral cancer.Arch Pharm Res. 2022 Aug;45(8):507-534. doi: 10.1007/s12272-022-01398-y. Epub 2022 Aug 20. Arch Pharm Res. 2022. PMID: 35987863 Review.
References
-
- Parish C. R., Freeman C., Hulett M. D. (2001) Heparanase: a key enzyme involved in cell invasion. Biochim. Biophys. Acta 1471, M99–108 - PubMed
-
- Dempsey L. A., Brunn G. J., Platt J. L. (2000) Heparanase, a potential regulator of cell-matrix interactions. Trends Biochem. Sci. 25, 349–351 - PubMed
-
- Ilan N., Elkin M., Vlodavsky I. (2006) Regulation, function, and clinical significance of heparanase in cancer metastasis and angiogenesis. Int. J. Biochem. Cell Biol. 38, 2018–2039 - PubMed
-
- Sanderson R. D., Yang Y., Kelly T., MacLeod V., Dai Y., Theus A. (2005) Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J. Cell. Biochem. 96, 897–905 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

