Group VIA PLA2 (iPLA2β) is activated upstream of p38 mitogen-activated protein kinase (MAPK) in pancreatic islet β-cell signaling
- PMID: 22194610
- PMCID: PMC3285329
- DOI: 10.1074/jbc.M111.285114
Group VIA PLA2 (iPLA2β) is activated upstream of p38 mitogen-activated protein kinase (MAPK) in pancreatic islet β-cell signaling
Abstract
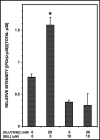
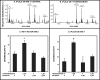
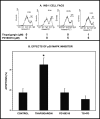
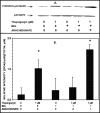
Group VIA phospholipase A(2) (iPLA(2)β) in pancreatic islet β-cells participates in glucose-stimulated insulin secretion and sarco(endo)plasmic reticulum ATPase (SERCA) inhibitor-induced apoptosis, and both are attenuated by pharmacologic or genetic reductions in iPLA(2)β activity and amplified by iPLA(2)β overexpression. While exploring signaling events that occur downstream of iPLA(2)β activation, we found that p38 MAPK is activated by phosphorylation in INS-1 insulinoma cells and mouse pancreatic islets, that this increases with iPLA(2)β expression level, and that it is stimulated by the iPLA(2)β reaction product arachidonic acid. The insulin secretagogue D-glucose also stimulates β-cell p38 MAPK phosphorylation, and this is prevented by the iPLA(2)β inhibitor bromoenol lactone. Insulin secretion induced by d-glucose and forskolin is amplified by overexpressing iPLA(2)β in INS-1 cells and in mouse islets, and the p38 MAPK inhibitor PD169316 prevents both responses. The SERCA inhibitor thapsigargin also stimulates phosphorylation of both β-cell MAPK kinase isoforms and p38 MAPK, and bromoenol lactone prevents both events. Others have reported that iPLA(2)β products activate Rho family G-proteins that promote MAPK kinase activation via a mechanism inhibited by Clostridium difficile toxin B, which we find to inhibit thapsigargin-induced β-cell p38 MAPK phosphorylation. Thapsigargin-induced β-cell apoptosis and ceramide generation are also prevented by the p38 MAPK inhibitor PD169316. These observations indicate that p38 MAPK is activated downstream of iPLA(2)β in β-cells incubated with insulin secretagogues or thapsigargin, that this requires prior iPLA(2)β activation, and that p38 MAPK is involved in the β-cell functional responses of insulin secretion and apoptosis in which iPLA(2)β participates.
Figures



Similar articles
-
Apoptosis of insulin-secreting cells induced by endoplasmic reticulum stress is amplified by overexpression of group VIA calcium-independent phospholipase A2 (iPLA2 beta) and suppressed by inhibition of iPLA2 beta.Biochemistry. 2004 Feb 3;43(4):918-30. doi: 10.1021/bi035536m. Biochemistry. 2004. PMID: 14744135 Free PMC article.
-
Studies of insulin secretory responses and of arachidonic acid incorporation into phospholipids of stably transfected insulinoma cells that overexpress group VIA phospholipase A2 (iPLA2beta ) indicate a signaling rather than a housekeeping role for iPLA2beta.J Biol Chem. 2001 Apr 20;276(16):13198-208. doi: 10.1074/jbc.M010423200. Epub 2001 Jan 22. J Biol Chem. 2001. PMID: 11278673
-
Characterization of FKGK18 as inhibitor of group VIA Ca2+-independent phospholipase A2 (iPLA2β): candidate drug for preventing beta-cell apoptosis and diabetes.PLoS One. 2013 Aug 20;8(8):e71748. doi: 10.1371/journal.pone.0071748. eCollection 2013. PLoS One. 2013. PMID: 23977134 Free PMC article.
-
Group VIA Ca2+-independent phospholipase A2 (iPLA2beta) and its role in beta-cell programmed cell death.Biochimie. 2010 Jun;92(6):627-37. doi: 10.1016/j.biochi.2010.01.005. Epub 2010 Jan 18. Biochimie. 2010. PMID: 20083151 Free PMC article. Review.
-
A link between endoplasmic reticulum stress-induced β-cell apoptosis and the group VIA Ca2+-independent phospholipase A2 (iPLA2β).Diabetes Obes Metab. 2010 Oct;12 Suppl 2(0 2):93-8. doi: 10.1111/j.1463-1326.2010.01270.x. Diabetes Obes Metab. 2010. PMID: 21029305 Free PMC article. Review.
Cited by
-
Group VIA phospholipase A2 mitigates palmitate-induced β-cell mitochondrial injury and apoptosis.J Biol Chem. 2014 May 16;289(20):14194-210. doi: 10.1074/jbc.M114.561910. Epub 2014 Mar 19. J Biol Chem. 2014. PMID: 24648512 Free PMC article.
-
Mechanism-based inhibition of iPLA2β demonstrates a highly reactive cysteine residue (C651) that interacts with the active site: mass spectrometric elucidation of the mechanisms underlying inhibition.Biochemistry. 2013 Jun 18;52(24):4250-63. doi: 10.1021/bi4004233. Epub 2013 Jun 10. Biochemistry. 2013. PMID: 23701211 Free PMC article.
-
Cytosolic and Calcium-Independent Phospholipases A2 Activation and Prostaglandins E2 Are Associated with Escherichia coli-Induced Reduction of Insulin Secretion in INS-1E Cells.PLoS One. 2016 Sep 15;11(9):e0159874. doi: 10.1371/journal.pone.0159874. eCollection 2016. PLoS One. 2016. PMID: 27631977 Free PMC article.
-
Group VIB Phospholipase A(2) promotes proliferation of INS-1 insulinoma cells and attenuates lipid peroxidation and apoptosis induced by inflammatory cytokines and oxidant agents.Oxid Med Cell Longev. 2012;2012:989372. doi: 10.1155/2012/989372. Epub 2012 Nov 11. Oxid Med Cell Longev. 2012. PMID: 23213352 Free PMC article.
-
Calcium-independent phospholipases A2 and their roles in biological processes and diseases.J Lipid Res. 2015 Sep;56(9):1643-68. doi: 10.1194/jlr.R058701. Epub 2015 May 28. J Lipid Res. 2015. PMID: 26023050 Free PMC article. Review.
References
-
- Mahler R. J., Adler M. L. (1999) Type 2 diabetes mellitus. Update on diagnosis, pathophysiology, and treatment. J. Clin. Endocr. Metab. 84, 1165–1171 - PubMed
-
- Hossain P., Kawar B., El Nahas M. (2007) Obesity and diabetes in the developing world. A growing challenge. N. Engl. J. Med. 356, 213–215 - PubMed
-
- Hotamisligil G. S., Shargill N. S., Spiegelman B. M. (1993) Adipose expression of tumor necrosis factor-α. Direct role in obesity-linked insulin resistance. Science 259, 87–91 - PubMed
-
- Ogden C. L., Carroll M. D., Curtin L. R., Lamb M. M., Flegal K. M. (2010) Prevalence of high body mass index in US children and adolescents. JAMA 303, 242–249 - PubMed
-
- Laing S. P., Swerdlow A. J., Slater S. D., Burden A. C., Morris A., Waugh N. R., Gatling W., Bingley P. J., Patterson C. C. (2003) Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 46, 760–765 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

